SGNTUC-019 Trial Final Results: Tucatinib + Trastuzumab Shows Durable Benefit in HER2-Mutant MBC at ESMO 2025
The final analysis of the SGNTUC-019 trial Phase II basket trial provides compelling evidence that the combination of tucatinib and trastuzumab offers durable clinical benefit and manageable toxicity in patients with previously treated HER2-mutant metastatic breast cancer (HER2-mut MBC)—a subgroup for which no HER2-targeted therapies are currently approved.
Background and Rationale
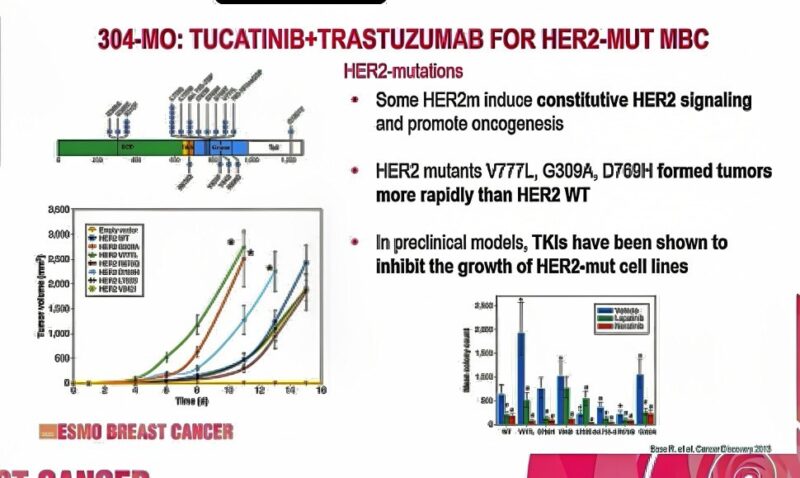
HER2 mutations represent a distinct molecular subset of metastatic breast cancer. These mutations, often found in the absence of HER2 amplification, are not amenable to conventional HER2-directed therapies developed for HER2-overexpressing disease. Tucatinib is a highly selective oral HER2 tyrosine kinase inhibitor currently approved in combination with trastuzumab and capecitabine for HER2-positive MBC. The SGNTUC-019 trial investigates the potential of tucatinib in HER2-mutant tumors—irrespective of amplification status—when paired with trastuzumab.
Patient Characteristics and Study Design
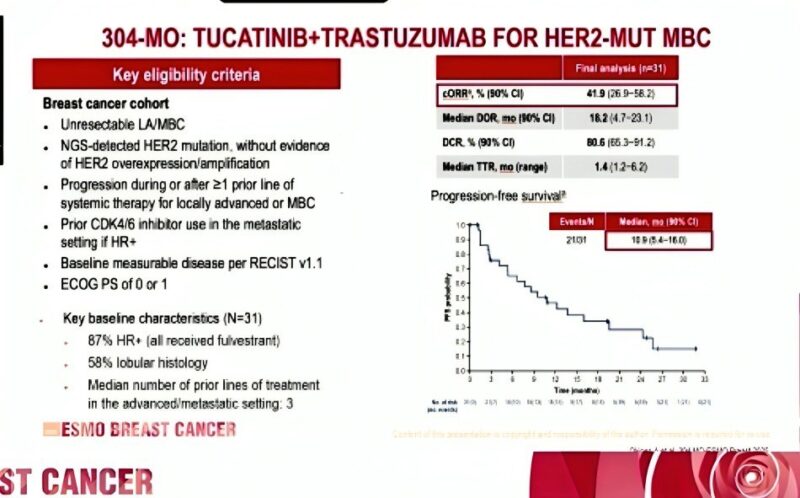
This HER2-mutant MBC cohort included 31 patients, most of whom had hormone receptor–positive disease (87%) and lobular histology (58%). All had previously received systemic therapy, with a median of three prior lines for locally advanced or metastatic disease. Patients with HR-positive tumors also received intramuscular fulvestrant. The regimen involved tucatinib at 300 mg twice daily and trastuzumab administered intravenously every three weeks, following an 8 mg/kg loading dose.
Efficacy Results
The final data reveal a confirmed objective response rate (cORR) of 41.9%, which includes two complete responses and 11 partial responses. Importantly, the median duration of response (DOR) extended to 18.2 months, up from 12.6 months reported in the primary analysis. Median progression-free survival (PFS) reached 10.9 months, while median overall survival (OS) was notably prolonged at 32.7 months, with the upper confidence interval not yet reached. These results suggest that a subset of patients derive long-lasting benefit from this chemotherapy-free regimen.
Read Full Abstract on ESMO Official Website
Safety and Tolerability
The combination continued to demonstrate a favorable safety profile with extended follow-up. The most frequent treatment-emergent adverse events were diarrhea (71%) and nausea (39%), largely consistent with the known toxicity profile of tucatinib. Grade ≥3 events occurred infrequently, with 13% experiencing severe diarrhea, 10% elevated ALT, and 10% hypertension. Only two patients (6%) discontinued tucatinib due to adverse events, and no treatment-related deaths were reported.
Clinical Implications
With limited treatment options available for HER2-mutant MBC and no approved HER2-targeted therapies in this setting, the results of SGNTUC-019 support the potential utility of tucatinib plus trastuzumab as a targeted approach. The regimen shows particular promise in patients with hormone receptor–positive and lobular subtypes, two groups often associated with endocrine resistance and therapeutic challenge.
These findings may influence clinical decision-making and future research directions, including efforts to secure regulatory approval and expand the role of HER2-directed therapies beyond HER2-amplified disease.
Conclusion
These final results confirm the durable efficacy and favorable safety profile of tucatinib plus trastuzumab in HER2-mutated MBC, a subset lacking approved targeted options. With a median OS exceeding 32 months, this regimen may represent a meaningful advance in managing this challenging disease subtype.
What They’re Saying: Reactions to SGNTUC-019 Trial at ESMO Breast 2025
Sara Tolaney,Chief, Division of Breast Oncology at DanaFarber Cancer Institute, shared on X
“Cristina Saura does an outstanding job discussing iza-bren, zani, and tucatinib +H”

Also Check Out Latest Updates from DESTINY-Breast06 Trial on Oncodaily
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
