
Sergio Cifuentes: Comparative Neoadjuvant and Adjuvant Studies in HER2-Positive Breast Cancer
Sergio Cifuentes, Cancer Research Project Manager at CENEIT México, posted on LinkedIn:
“HER2-positive breast cancer is characterized by the amplification or overexpression of the HER2 protein, a tyrosine kinase receptor that promotes cell growth and division.1 This biological feature gives tumors a more aggressive behavior and a higher propensity for metastasis.1 Historically, HER2-positive breast cancer was associated with a poor prognosis due to its aggressive nature, but the development of therapies specifically targeting the HER2 pathway has dramatically improved clinical outcomes (1). This subtype accounts for approximately 15-20% of all invasive breast cancers (2).
Neoadjuvant therapy is administered before surgery in patients with locally advanced breast cancer (clinical stage IIB-IIIC) and operable tumors larger than 2 cm (1). Its goals include reducing tumor size to facilitate breast-conserving surgery, assessing in vivo response to systemic therapy, and potentially eradicating micrometastatic disease.
Pathological complete response (pCR), defined as the absence of residual invasive cancer in the breast and sampled lymph nodes, is a critical endpoint in neoadjuvant trials for HER2-positive breast cancer and has been correlated with better long-term outcomes, especially in the hormone receptor-negative subgroup (8).
Adjuvant therapy is administered after surgery to eliminate any remaining microscopic cancer cells, reduce the risk of local and distant recurrence, and ultimately improve disease-free survival and overall survival (5). Key endpoints in adjuvant trials include invasive disease-free survival (IDFS) and overall survival (OS).
The treatment of HER2-positive breast cancer has undergone remarkable evolution with the development of increasingly specific therapies.
The first major breakthrough was the creation and approval of trastuzumab, a humanized monoclonal antibody that binds to the extracellular domain of the HER2 protein, inhibiting signaling, angiogenesis, and proliferation (1) Subsequently, other HER2-targeted agents with different mechanisms of action were developed, such as pertuzumab (another monoclonal antibody that binds to a different domain of HER2, preventing HER2-HER3 dimerization), lapatinib (a small molecule tyrosine kinase inhibitor that reversibly inhibits both HER1 and HER2), trastuzumab emtansine (T-DM1), an antibody-drug conjugate that delivers a cytotoxic payload directly to HER2-positive cells, and the more recently investigated trastuzumab deruxtecan (T-DXd), another antibody-drug conjugate with a higher drug-to-antibody ratio and a different cytotoxic payload (1).
The purpose of this report is to provide a comprehensive comparative analysis of the main neoadjuvant and adjuvant clinical trials that have shaped the treatment landscape for HER2-positive breast cancer. It will focus on summarizing the study designs, treatment regimens, primary endpoints, and significant findings of these trials. Furthermore, the report will incorporate and analyze the preliminary results of the DESTINY-Breast11 trial, which investigates the role of trastuzumab deruxtecan (T-DXd) in the neoadjuvant setting, and discuss its potential impact on current treatment paradigms.
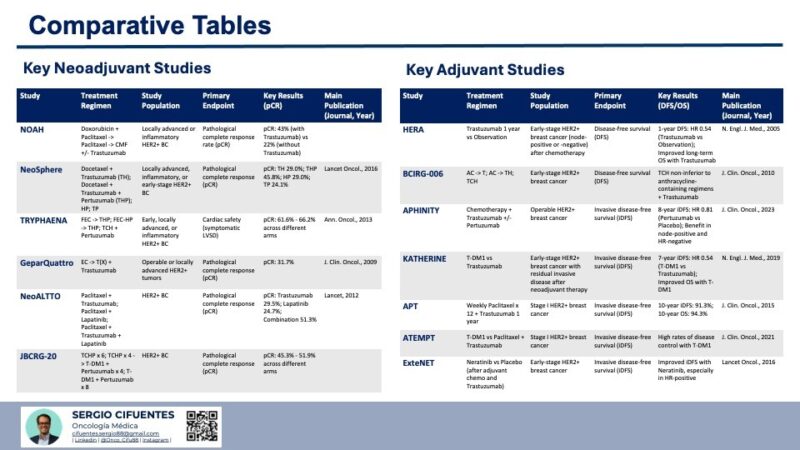
Key Neoadjuvant Studies in HER2-Positive Breast Cancer:
Neoadjuvant therapy in HER2-positive breast cancer has proven to be an effective strategy for improving surgical outcomes and achieving pathological complete responses. Several key studies have contributed to defining the current standard of care.
- The NOAH trial was pivotal in establishing the benefit of adding trastuzumab to neoadjuvant chemotherapy in locally advanced HER2-positive breast cancer. The significant increase in pCR and improvement in EFS demonstrated the efficacy of targeting HER2 in the preoperative setting, representing a major advancement in the treatment of this aggressive form of breast cancer (1). Prior to the NOAH trial, the standard neoadjuvant treatment for locally advanced breast cancer primarily consisted of chemotherapy. This study provided the first strong evidence that incorporating a HER2-targeted agent like trastuzumab upfront could significantly enhance the response to chemotherapy, leading to better tumor regression and potentially improved long-term outcomes. The substantial difference in pCR rates between the two arms clearly highlighted the importance of HER2 inhibition in this context (17)
- The NeoSphere trial provided compelling evidence for the significant improvement in pCR rates with the addition of pertuzumab to trastuzumab and docetaxel in the neoadjuvant setting for HER2-positive breast cancer (18). This study demonstrated the synergistic effect of dual HER2 blockade and supported the incorporation of pertuzumab into standard neoadjuvant regimens. By comparing different combinations of HER2-targeted therapies with chemotherapy, NeoSphere clearly showed that the simultaneous blockade of two distinct epitopes on the HER2 receptor with trastuzumab and pertuzumab, when combined with docetaxel, resulted in a significantly higher proportion of patients achieving a pathological complete response. This suggested that a more comprehensive blockade of the HER2 signaling pathway led to better antitumor activity in the preoperative setting (22).
- The TRYPHAENA trial established the cardiac safety and efficacy of neoadjuvant regimens containing pertuzumab and trastuzumab with both anthracycline-containing and anthracycline-free chemotherapy backbones . The high pCR rates achieved across all arms, along with acceptable cardiac safety profiles, provided clinicians with more options to tailor neoadjuvant therapy based on individual patient characteristics and risk factors. Given the known cardiotoxicity associated with anthracyclines, demonstrating that an anthracycline-free regimen (TCH + Pertuzumab) could achieve high pCR rates comparable to anthracycline-containing regimens was a significant finding. This offered a valuable alternative for patients who might be at higher risk for cardiac complications, allowing for effective HER2-targeted neoadjuvant therapy without compromising efficacy .
- The GeparQuattro trial confirmed that combining trastuzumab with standard anthracycline- and taxane-based neoadjuvant chemotherapy resulted in a significantly higher pCR rate in HER2-positive breast cancer compared to chemotherapy alone . This large study further solidified the role of trastuzumab as a crucial component of neoadjuvant treatment for this breast cancer subtype. Building upon the findings of earlier trials, GeparQuattro provided more robust evidence of the substantial benefit of adding trastuzumab to conventional neoadjuvant chemotherapy regimens in patients with HER2-positive breast cancer. The significant difference in pCR rates clearly demonstrated the efficacy of HER2-targeted therapy in achieving greater tumor regression before surgery .
- The NeoALTTO trial demonstrated that dual HER2 blockade using a combination of trastuzumab and lapatinib, which target the HER2 receptor through different mechanisms, resulted in a significantly higher pCR rate compared to trastuzumab alone in the neoadjuvant setting (8). This highlighted the potential of combining different HER2-targeted therapies to achieve greater antitumor activity. While pertuzumab also provides dual HER2 blockade by preventing dimerization, NeoALTTO explored an alternative strategy, suggesting that inhibiting HER2 signaling at both the extracellular and intracellular levels could be particularly effective in inducing tumor regression before surgery (21).
- The JBCRG-20 trial explored the integration of trastuzumab emtansine (T-DM1), an antibody-drug conjugate, into neoadjuvant treatment regimens for HER2-positive breast cancer (8). The high pCR rates observed in the different arms suggested that T-DM1 could be an effective component of neoadjuvant therapy, potentially offering an alternative or complementary approach to traditional chemotherapy regimens. Given the proven efficacy of T-DM1 in the adjuvant setting for patients with residual disease (as demonstrated in the KATHERINE trial), investigating its role earlier in the treatment sequence was a logical next step to further optimize neoadjuvant strategies(25).
Other relevant studies include DESTINY-Breast01, a pivotal phase II trial in heavily pretreated HER2-positive metastatic breast cancer that demonstrated remarkable and durable antitumor activity with trastuzumab deruxtecan (T-DXd), with an objective response rate of 60.9% and a median progression-free survival of 16.4 months. This trial highlighted the potential of T-DXd as a highly effective HER2-targeted agent. Additionally, the EA1211/DIRECT trial (26) is ongoing for patients with early HER2-positive breast cancer with the aim of validating FDG-PET/CT scanning as an interim imaging biomarker to predict pathological complete response (pCR) to pertuzumab-based neoadjuvant chemotherapy.
The NeoCARHP trial 28 compares neoadjuvant TCHP (docetaxel, carboplatin, trastuzumab, and pertuzumab) versus THP (paclitaxel, trastuzumab, and pertuzumab) in patients with HER2-positive breast cancer. Finally, the NeoATP trial (29) is a phase II trial evaluating neoadjuvant trastuzumab and pyrotinib (another reversible HER2 tyrosine kinase inhibitor) plus anthracycline-free chemotherapy for locally advanced HER2-positive breast cancer.
The evolution of neoadjuvant therapy for HER2-positive breast cancer has been characterized by a progressive intensification of HER2 blockade, initially based on trastuzumab alone, then with the addition of pertuzumab and the exploration of other dual-blockade strategies. There is also a growing emphasis on minimizing the use of anthracyclines while maintaining high efficacy and on investigating the role of highly active antibody-drug conjugates like T-DM1 and the emerging potential of T-DXd in this setting.
Key Adjuvant Studies in HER2-Positive Breast Cancer:
Adjuvant therapy has been shown to significantly improve outcomes in patients with early-stage HER2-positive breast cancer after surgery. Several pivotal clinical trials have established the current standard of care.
- The HERA trial was a landmark study that established one year of adjuvant trastuzumab as the standard of care for patients with early-stage HER2-positive breast cancer after chemotherapy . This study demonstrated a substantial and sustained reduction in the risk of recurrence and death, regardless of age, nodal status, and hormone receptor status . Before the HERA trial, the role of trastuzumab was mainly limited to the treatment of metastatic disease. This trial definitively showed its benefit in the adjuvant setting, preventing the development of distant metastases and improving long-term survival for patients with early-stage HER2-positive breast cancer. The clear and consistent improvement in outcomes with one year of trastuzumab led to its rapid adoption as a cornerstone of adjuvant therapy for this disease .
- The BCIRG-006 trial provided crucial evidence that effective adjuvant therapy for early-stage HER2-positive breast cancer could be achieved using a non-anthracycline-containing chemotherapy regimen (TCH) in combination with trastuzumab. This offered a valuable alternative for patients who might have contraindications to anthracyclines or concerns about the potential long-term cardiotoxicity associated with these agents. While anthracycline-based chemotherapy regimens were historically a standard component of adjuvant treatment for early-stage breast cancer, BCIRG-006 demonstrated that similar efficacy could be obtained in HER2-positive disease with the TCH regimen when combined with trastuzumab. This finding was particularly important for allowing clinicians to tailor treatment to individual patient risk factors and comorbidities, offering a less cardiotoxic option without compromising the effectiveness of HER2-targeted therapy .
- The APHINITY trial demonstrated that the addition of pertuzumab to adjuvant chemotherapy and trastuzumab provided a statistically significant benefit in terms of invasive disease-free survival (iDFS) for patients with high-risk early-stage HER2-positive breast cancer, particularly those with node-positive disease . This supported the use of dual HER2 blockade in the adjuvant setting for patients at higher risk of recurrence. Building upon the success of dual HER2 blockade in metastatic and neoadjuvant settings, APHINITY aimed to determine if this approach would also improve outcomes in the adjuvant treatment of early-stage HER2-positive breast cancer. The significant improvement in iDFS, especially in the node-positive subgroup, indicated that the addition of pertuzumab could further reduce the risk of disease recurrence in higher-risk patients after surgery.
- The KATHERINE trial established a new standard of care for patients with early-stage HER2-positive breast cancer who have residual invasive disease after completing neoadjuvant chemotherapy and HER2-targeted therapy . Adjuvant treatment with T-DM1 resulted in a statistically significant and clinically relevant improvement in both invasive disease-free survival (iDFS) and overall survival (OS) compared to trastuzumab alone . This trial addressed a critical clinical need in the management of HER2-positive breast cancer by identifying a more effective adjuvant treatment strategy for patients at higher risk of recurrence due to the presence of residual disease after neoadjuvant therapy. The substantial improvements in both disease-free and overall survival with T-DM1 highlighted the importance of response-guided adjuvant therapy and the efficacy of antibody-drug conjugates in this setting.
- The APT trial demonstrated that a less intensive adjuvant regimen consisting of weekly paclitaxel for a short duration followed by one year of trastuzumab could lead to excellent long-term outcomes in patients with small, node-negative, stage I HER2-positive breast cancer (14). This provided evidence for potential de-escalation of therapy in this low-risk population. This trial challenged the traditional approach of using more aggressive chemotherapy regimens in all patients with HER2-positive breast cancer, suggesting that a more tailored approach based on risk stratification could lead to excellent outcomes with less toxicity in certain low-risk cases (33).
- The ATEMPT trial further explored the possibility of reducing the intensity of adjuvant therapy in low-risk HER2-positive breast cancer by evaluating the efficacy and safety of trastuzumab emtansine (T-DM1) as an alternative to the standard paclitaxel and trastuzumab regimen (35) The favorable results observed with T-DM1 suggested that it could be a valuable option, potentially with a different toxicity profile. This trial aimed to identify alternative, potentially less toxic but highly effective, adjuvant treatment options for patients with low-risk, early-stage HER2-positive breast cancer. The comparison with the well-established APT regimen provided important information on the relative efficacy and safety of T-DM1 in this specific patient population (33).
- The ExteNET trial explored the concept of extended adjuvant therapy beyond the standard one year of trastuzumab in HER2-positive breast cancer. The finding of an improvement in iDFS with neratinib suggested that prolonged HER2 inhibition could provide additional benefit in reducing the risk of late recurrences in certain patients. This trial addressed the clinical challenge of late recurrences in HER2-positive breast cancer by investigating whether extending HER2-targeted therapy with an oral tyrosine kinase inhibitor could further improve long-term outcomes after completing standard adjuvant treatment.
Adjuvant treatment for HER2-positive breast cancer has become increasingly refined, evolving from chemotherapy followed by trastuzumab to the incorporation of pertuzumab for higher-risk disease and T-DM1 for patients with residual disease after neoadjuvant treatment. There is also a growing understanding of the potential for de-escalation in low-risk early-stage disease.
(Preliminary) Results from Destiny Breast 11:
DESTINY-Breast11 is a global, multicenter, randomized, open-label phase 3 trial. The trial is evaluating the efficacy and safety of neoadjuvant ENHERTU (trastuzumab deruxtecan) monotherapy or ENHERTU followed by THP (paclitaxel, trastuzumab, pertuzumab) compared to the standard of care regimen of ddAC (dose-dense doxorubicin and cyclophosphamide) followed by THP in patients with high-risk HER2-positive early-stage breast cancer (lymph node positive [N1-3] or with a primary tumor stage T3-4) .
Patients were randomized 1:1:1 to one of three neoadjuvant treatment arms:
- Arm A: Eight cycles of ENHERTU monotherapy (5.4 mg/kg).
- Arm B: Four cycles of ENHERTU (5.4 mg/kg) followed by four cycles of THP (paclitaxel, trastuzumab, pertuzumab).
- Arm C: Four cycles of ddAC followed by four cycles of THP (standard of care)
The primary endpoint of DESTINY-Breast11 is the pathological complete response rate (pCR), defined as the absence of invasive disease in the breast and lymph nodes, as assessed by blinded independent central review (BICR) . Secondary endpoints include event-free survival (EFS), invasive disease-free survival, overall survival, pharmacokinetics, immunogenicity, and safety.. The trial enrolled 927 patients across multiple sites in Asia, Europe, North America, and South America.
DESTINY-Breast11 is the first phase 3 trial specifically designed to evaluate the efficacy and safety of trastuzumab deruxtecan (ENHERTU) in the neoadjuvant treatment of high-risk HER2-positive early-stage breast cancer, comparing it to the current standard of care. This trial has the potential to establish a new treatment paradigm in this setting. Given the impressive activity of ENHERTU observed in HER2-positive metastatic breast cancer, investigating its role in the neoadjuvant treatment of high-risk early-stage disease is a logical and important step. This trial aims to determine if ENHERTU-based regimens can improve the pathological complete response rate, a key indicator of long-term outcomes, and potentially offer a more effective treatment option for patients with a higher risk of recurrence.
Preliminary results from DESTINY-Breast11 showed that ENHERTU followed by THP (Arm B) demonstrated a statistically significant and clinically relevant improvement in the pathological complete response (pCR) rate compared to the standard of care regimen of ddAC followed by THP (Arm C) when used in the neoadjuvant setting (before surgery) in patients with high-risk, locally advanced HER2-positive early-stage breast cancer.
Event-free survival (EFS) data showed an early positive trend favoring ENHERTU followed by THP compared to the standard of care, but were not mature at the time of the analysis. The trial will continue to follow EFS. ENHERTU followed by THP also showed an improved safety profile compared to the standard of care regimen (ddAC-THP). The safety profiles of ENHERTU and THP were consistent with their known profiles, and no new safety concerns were identified in the combination arm.
Rates of interstitial lung disease (ILD) were similar in both arms of the trial (ENHERTU followed by THP and ddAC followed by THP), as determined by an independent adjudication committee. Following a recommendation by the Independent Data Monitoring Committee, patient enrollment in the arm evaluating ENHERTU monotherapy was closed after a previous interim efficacy assessment . The preliminary results of DESTINY-Breast11 are highly promising, suggesting that ENHERTU followed by THP could become a new standard of care in the neoadjuvant treatment of high-risk HER2-positive early-stage breast cancer.
The significant improvement in pCR rate, along with a more favorable safety profile compared to the anthracycline-containing standard of care, indicates a potential for better tumor control and a more favorable toxicity profile for patients. The finding of a statistically significant and clinically relevant improvement in pCR rate with the ENHERTU-containing regimen compared to the standard of care is a major advancement in the neoadjuvant treatment of high-risk HER2-positive early-stage breast cancer.
This suggests that ENHERTU, when used in this sequence, is more effective at eradicating the tumor before surgery. The additional finding of an improved safety profile compared to the standard anthracycline-based regimen is particularly important, as it indicates that this increased efficacy could be achieved with less toxicity for patients, potentially leading to better tolerability and quality of life during treatment. The early positive trend observed in event-free survival (EFS) with ENHERTU followed by THP is encouraging and warrants further follow-up as the data mature.
If this trend holds with longer follow-up, it would suggest that the improvement in pCR observed with this regimen translates into a sustained benefit in preventing disease recurrence. While pathological complete response (pCR) is a valuable surrogate endpoint in neoadjuvant trials, the ultimate goal is to improve long-term outcomes for patients.
The early indication of a positive trend in EFS with the ENHERTU-containing regimen suggests that the initial benefits observed with pCR might be maintained over time, leading to a reduced risk of disease recurrence and potentially improved survival. However, it is crucial to await mature EFS data to confirm this potential long-term benefit. The similar rates of interstitial lung disease (ILD) observed in both the ENHERTU followed by THP arm and the standard of care arm are reassuring from a safety perspective.
Given the known risk of ILD associated with ENHERTU, this finding suggests that the use of ENHERTU in this neoadjuvant combination does not appear to significantly increase the risk of this serious adverse event compared to the standard anthracycline-based regimen. Interstitial lung disease (ILD) is a recognized potential complication of ENHERTU treatment, and it is important to carefully evaluate its incidence in clinical trials. The finding of similar rates of ILD in the ENHERTU-containing arm compared to the standard of care arm in DESTINY-Breast11 provides important safety information and suggests that the risk of this adverse event may be manageable in this neoadjuvant setting.
The early closure of the ENHERTU monotherapy arm in DESTINY-Breast11 after an interim efficacy assessment likely indicates that this treatment approach was not as effective as the combination regimen of ENHERTU followed by THP or the standard of care in achieving a high pathological complete response rate in this high-risk patient population. Clinical trials often include interim analyses to evaluate the efficacy and safety of investigational treatments.
The decision to close an arm of a trial early is typically made when interim data suggest that the treatment is unlikely to meet its primary endpoint or if there are safety concerns. In the case of the ENHERTU monotherapy arm in DESTINY-Breast11, the early closure likely reflects a determination that this approach was not demonstrating a sufficient level of efficacy compared to the other treatment arms in this specific patient population with high-risk HER2-positive early-stage breast cancer.
Discussion:
The evolving landscape of neoadjuvant and adjuvant therapy for HER2-positive breast cancer reflects an ongoing pursuit of more effective and less toxic treatments. Neoadjuvant therapy has progressed from chemotherapy alone (as a historical control in NOAH) to the integration of trastuzumab, the addition of pertuzumab for dual HER2 blockade (NeoSphere, TRYPHAENA), and the exploration of other dual-targeting strategies (NeoALTTO).
The preliminary results from DESTINY-Breast11 suggest a potential further advancement with the incorporation of the highly active antibody-drug conjugate T-DXd (ENHERTU) into neoadjuvant regimens for high-risk early-stage disease. In the adjuvant setting, the HERA trial established the fundamental role of one year of trastuzumab. Subsequent trials like BCIRG-006 demonstrated the feasibility of non-anthracycline-containing regimens with trastuzumab. APHINITY extended the benefits of dual HER2 blockade to the adjuvant setting for higher-risk patients.
The KATHERINE trial highlighted the importance of response-guided adjuvant therapy, establishing T-DM1 as the standard for patients with residual disease after neoadjuvant treatment. Furthermore, trials like APT and ATEMPT have explored the potential for de-escalation in low-risk early-stage disease.
The preliminary findings from DESTINY-Breast11 are very promising for the neoadjuvant treatment of high-risk HER2-positive early-stage breast cancer. The statistically significant and clinically relevant improvement in pCR rate observed with ENHERTU followed by THP compared to the standard anthracycline-containing regimen suggests a potential new standard of care. This regimen also demonstrated an improved safety profile, which is a crucial consideration in the neoadjuvant setting where the goal is to achieve the best possible outcome before surgery while minimizing treatment-related toxicities. The early positive trend in event-free survival further supports the potential of this ENHERTU-based approach to translate into long-term benefits for patients.
While the preliminary results of DESTINY-Breast11 are encouraging, further research and mature data are needed to fully understand the long-term impact of this neoadjuvant regimen on event-free survival and overall survival. Clinicians will need to carefully consider patient selection for ENHERTU-based neoadjuvant therapy, taking into account factors such as tumor characteristics, nodal status, and the potential risks associated with ENHERTU, including interstitial lung disease.2 Ongoing research continues to explore the optimal sequence and duration of HER2-targeted therapies in both the neoadjuvant and adjuvant settings.
Trials are investigating the role of T-DXd in the adjuvant treatment for patients with residual disease after neoadjuvant therapy (e.g., DESTINY-Breast05 ) and exploring the potential of chemotherapy-free regimens in certain patient populations.6 The field is also focused on refining de-escalation strategies in low-risk early-stage disease and identifying biomarkers that can help personalize treatment decisions and predict response to specific therapies .
The evolving landscape of neoadjuvant and adjuvant therapy for HER2-positive breast cancer reflects a continuous drive towards more effective and less toxic treatments. The promising preliminary results of DESTINY-Breast11 highlight the potential of trastuzumab deruxtecan (ENHERTU) to further improve outcomes in high-risk early-stage disease. As data from this and other ongoing trials mature, they will undoubtedly continue to refine our understanding of the optimal management of HER2-positive breast cancer across all stages.”
More posts featuring Sergio Cifuentes on OncoDaily.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
