
Vimseltinib (Romvimza): What patients need to know?
Vimseltinib is a newly FDA-approved treatment for tenosynovial giant cell tumor (TGCT), a rare type of tumor that affects the joints and tendons. Approved on February 14, 2025, Vimseltinib is a promising oral medication that provides an innovative way to manage TGCT, especially when surgery is not an option due to the risk of severe complications. This article explains how Vimseltinib works, its dosage, potential side effects, and what patients may experience during treatment.
What Is Vimseltinib and How Does It Work?
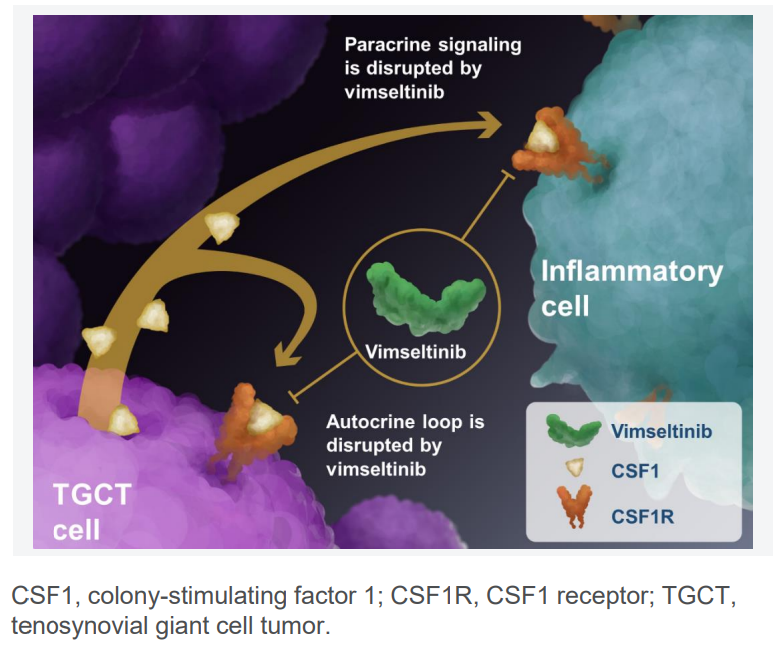
Vimseltinib, marketed under the brand name Romvimza, is a targeted oral medication known as a selective kinase inhibitor. It specifically targets the colony-stimulating factor 1 receptor (CSF1R), which plays a crucial role in the growth and survival of certain immune cells called macrophages. These cells can contribute to the growth of tumors, such as those seen in TGCT.
What makes Vimseltinib unique is its design as a “switch-control inhibitor.” Unlike other CSF1R inhibitors, it binds to a specific region on the CSF1R receptor, blocking the signaling pathways that help tumors grow and spread. This selective targeting allows Vimseltinib to reduce tumor size and improve symptoms without causing unnecessary side effects in healthy tissue.
What Cancers Is Vimseltinib Approved to Treat?
As of now, Vimseltinib is FDA-approved specifically for the treatment of symptomatic TGCT in adults when surgery is not an option due to the risk of severe morbidity or functional limitations. While TGCT is the only cancer for which Vimseltinib has received regulatory approval, ongoing research is exploring its potential to treat other cancers that involve CSF1R-driven tumor growth.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Vimseltinib was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
How Effective Is Vimseltinib?
The effectiveness of Vimseltinib was demonstrated in the MOTION trial, a phase 3 clinical study that evaluated the drug’s impact on patients with TGCT who were not candidates for surgery. In this trial, patients who received Vimseltinib showed a significantly higher objective response rate (40%) compared to those who received a placebo (0%). Additionally, Vimseltinib improved symptoms such as joint pain, stiffness, and reduced range of motion, contributing to a better quality of life for patients.
What Is Tenosynovial Giant Cell Tumor (TGCT)?
Tenosynovial Giant Cell Tumor (TGCT) is a rare, non-cancerous tumor that affects the thin tissue lining the joints and tendons, called the synovium. While it’s not cancer, TGCT can grow and cause pain, swelling, and joint problems over time.
There are two main types:
- Localized TGCT (also known as giant cell tumor of the tendon sheath): This is more common and usually affects small joints, especially in the fingers or hands. It often appears as a painless lump and grows slowly.
- Diffuse TGCT (also known as pigmented villonodular synovitis or PVNS): This type is less common and usually affects larger joints like the knee. It can cause more pain, stiffness, and joint damage if not treated.
TGCT happens when a genetic change causes the body to make too much of a substance called CSF-1, which attracts immune cells that form the tumor. The exact cause is unknown, and it can affect both men and women, usually between ages 25 and 50.
What Are the Symptoms of TGCT?
Symptoms may include:
- Joint pain or tenderness
- Swelling and stiffness
- A feeling of instability in the joint
- A lump or mass near the joint
These symptoms can look like other conditions, so doctors may use imaging (like MRI or X-rays) and sometimes a biopsy to confirm the diagnosis.
How Is TGCT Treated?
The main treatment is surgery to remove the tumor. However, in diffuse TGCT, the tumor can come back after surgery. In these cases, other treatments may be used, such as:
- Radiation therapy
- Targeted medications like pexidartinib (Turalio) or imatinib (Gleevec), which help shrink the tumor when surgery isn’t possible or if it keeps coming back.
Early diagnosis and treatment can help prevent joint damage and improve movement.
What Other Trials Are Ongoing?
Beyond TGCT, Vimseltinib is being studied in other clinical trials to evaluate its potential in treating other cancers, including chronic graft-versus-host disease (cGVHD), a complication of stem cell transplants. Ongoing research will help clarify its broader role in oncology.

You can read about Bone cancer: What patients should know on OncoDaily.
Vimseltinib Dosage and Administration Guide for TGCT Patient
Vimseltinib, sold under the brand name Romvimza, is prescribed for adults with symptomatic tenosynovial giant cell tumor (TGCT) when surgery might cause serious mobility issues. The recommended dose is 30 mg taken by mouth twice a week, with at least 72 hours between doses. The medication may be taken with or without food.
The capsules must be swallowed whole—not opened, crushed, or chewed. Patients are instructed to follow the weekly schedule provided in the blister pack and take their doses on the same days each week.
If a dose is missed and less than 48 hours have passed, it should be taken as soon as remembered. If more than 48 hours have passed, the missed dose should be skipped and the next dose taken on the usual day. If vomiting occurs within 30 minutes of taking the medication, the dose should be repeated. If more than 30 minutes have passed, no extra dose is needed.
Romvimza should be stored in its original blister packaging at room temperature (20–25°C or 68–77°F) and not transferred to other containers.
Vimseltinib Side Effects and Management
Like all medications, Vimseltinib can cause some side effects, but not everyone will experience them. The most common side effects are usually mild to moderate, and they can be managed with a few simple steps.
Common Side Effects
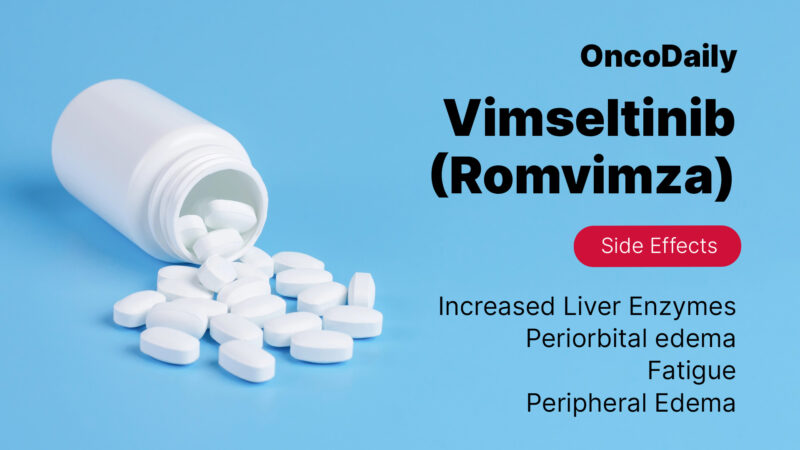
Fatigue is a common side effect, and many patients feel more tired than usual while taking Vimseltinib. This fatigue is generally manageable with proper rest, staying hydrated, and balancing activity with rest. If fatigue becomes overwhelming, patients should speak with their healthcare provider to find ways to better manage it.
A rash may also develop in some patients, but it is usually mild. In these cases, doctors may recommend treatments like creams or antihistamines to ease the discomfort. The rash often goes away as the body adjusts to the medication.
Swelling around the eyes, known as periorbital edema, is another side effect that may occur. This swelling can typically be managed by adjusting the medication dose. Doctors will monitor the patient and make changes if necessary to reduce swelling and improve comfort.
Elevated liver enzymes can occur during treatment with Vimseltinib. Because of this, patients will likely undergo regular blood tests to monitor liver function. If liver enzyme levels rise too high, doctors may adjust the dose or temporarily stop treatment to protect the liver.
Higher cholesterol levels are another side effect that some patients may experience. If this happens, doctors may recommend changes to the patient’s diet or prescribe medications to help manage cholesterol levels.
Less common side effects
In addition to these common side effects, some patients may experience less common issues. Gastrointestinal problems such as nausea or diarrhea may occur, but they can often be managed with hydration and medications to ease symptoms. Headaches and muscle or joint pain are also possible, and they can usually be treated with over-the-counter pain relievers or by adjusting activity levels. Some patients may notice an increase in blood pressure, a condition called hypertension.
If this occurs, doctors will monitor the patient’s blood pressure and, if necessary, provide treatment to keep it under control.
Although these side effects may sound concerning, most can be managed with the help of a healthcare team. By adjusting the treatment plan or providing supportive care, doctors can help minimize discomfort and ensure the best possible outcome. Patients need to communicate openly with their healthcare provider about any side effects they experience so that they can receive timely and appropriate care.
What Should You Avoid During Treatment?
While taking Vimseltinib, there are a few important things to avoid to make sure the medication works effectively and to minimize any potential risks.
First, it’s important to avoid grapefruit and grapefruit juice. These can interfere with how Vimseltinib is broken down in the body, potentially affecting how well the medication works. It’s best to steer clear of these while undergoing treatment.
Alcohol should also be avoided as it can increase the risk of liver toxicity. Drinking alcohol while on Vimseltinib may put extra stress on the liver, so it’s wise to limit or completely avoid alcohol during treatment.
Pregnancy is another important consideration. Vimseltinib can harm a developing fetus, so it should not be taken during pregnancy. Patients should use effective contraception during treatment and for some time after stopping the medication to ensure there is no risk to a potential pregnancy.
Finally, patients should avoid breaking or chewing the capsules. This is important because Vimseltinib is designed to be absorbed by the body in a specific way, and altering the form of the capsules may affect how the medication works. Capsules should be swallowed whole to ensure they provide the intended benefits.
By following these guidelines, patients can help ensure their treatment with Vimseltinib is as safe and effective as possible. It’s always a good idea to discuss any concerns with a healthcare provider who can provide additional advice and support throughout the treatment process.
Long-Term Outlook with Vimseltinib
Vimseltinib shows significant promise in the short term for reducing TGCT symptoms and improving function. While it is not a cure for TGCT, it offers hope for managing this rare tumor, especially for patients who cannot undergo surgery. Ongoing studies will provide more information on its long-term effectiveness.
Looking Ahead – The Future of Treatment
Vimseltinib represents an exciting advance in the treatment of TGCT. As research continues, it may be explored for use in additional cancers that involve CSF1R-driven tumor growth. The potential for combination therapies with other cancer treatments may further enhance its effectiveness.
For patients diagnosed with TGCT, Vimseltinib offers a new, targeted approach to treatment, with the hope of reducing tumor size and improving quality of life. Speak with your healthcare provider to learn more about how Vimseltinib may fit into your treatment plan.
If you are a health care provider, here is the professional version.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
