
Bladder Cancer Treatment: The Game-Changing Role of Antibody-Drug Conjugates (ADCs)
Bladder cancer is a disease in which malignant (cancer) cells form in the tissues of the bladder, the organ that stores urine. It most commonly begins in the urothelial cells lining the bladder wall. The primary types include urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma.Bladder cancer often presents with blood in the urine, frequent urination, or pain during urination. Risk factors include smoking, exposure to industrial chemicals, chronic bladder inflammation, and age (more common in older adults). Antibody-drug conjugates (ADCs) have emerged as a promising new approach, offering a more targeted and effective treatment strategy with fewer side effects.

You Can Read More About Bladder Cancer on Oncodaily
What Are Antibody-Drug Conjugates (ADCs) and How Are They Different from Standard Chemotherapy?
Antibody-Drug Conjugates (ADCs) are a new type of cancer treatment that combine a targeted antibody with a chemotherapy drug. They work by specifically seeking out cancer cells and delivering the drug directly inside the tumor, minimizing damage to healthy cells. By delivering chemotherapy directly into cancer cells, ADCs enhance effectiveness and minimize the toxic effects of traditional chemotherapy.

Standard chemotherapy works by attacking all fast-growing cells, including both cancerous and healthy cells. This is why chemotherapy often causes hair loss, nausea, and immune suppression—because it affects not just the tumor but also healthy tissues like hair follicles, the digestive system, and bone marrow.In contrast, ADCs are highly targeted. They use an antibody that specifically recognizes a protein found on cancer cells, delivering the chemotherapy drug directly inside the tumor. This reduces damage to healthy cells, leading to fewer side effects and a more precise treatment approach.
With chemotherapy, the drug circulates through the entire body, attacking cancer wherever it’s found but also affecting normal tissues. ADCs work differently—they only become active once they bind to the cancer cell. Once attached, the ADC is absorbed into the tumor, where it releases its toxic drug directly inside the cancer cell. This approach increases the drug’s effectiveness while minimizing harm to healthy tissues.
Standard Treatment for Metastatic Bladder Cancer Before Antibody-Drug Conjugates
Before the introduction of antibody-drug conjugates (ADCs), the primary treatment for metastatic bladder cancer was chemotherapy. The most commonly used chemotherapy regimen involved a combination of cisplatin and gemcitabine. These drugs aimed to reduce the size of the tumor and slow the progression of the cancer, though they often came with significant side effects. In cases where chemotherapy was ineffective or the cancer recurred, doctors might have turned to other treatments like immunotherapy, which works by boosting the body’s immune system to target and fight cancer cells.
Who Should Receive ADCs?
Antibody-Drug Conjugates (ADCs) are not for all cancer patients. They are usually given to specific groups based on cancer stage, previous treatments, and tumor characteristics.
Patients with advanced or metastatic bladder cancer are the primary candidates for ADC therapy. These drugs are used when the cancer has spread beyond the bladder and standard treatments have failed. ADCs like Enfortumab Vedotin (Padcev) and Sacituzumab Govitecan (Trodelvy) have shown effectiveness in slowing disease progression and improving survival in these patients.
Most patients receiving ADCs have already tried chemotherapy or immunotherapy. Enfortumab Vedotin, for example, is approved for patients who have been treated with both chemotherapy and immune checkpoint inhibitors like pembrolizumab (Keytruda). Sacituzumab Govitecan is an option for those who have undergone at least two prior treatments, including chemotherapy, but whose cancer continues to progress.
You Can Read More About Enfortumab Vedotin on Oncodaily
Since ADCs work by targeting specific proteins on cancer cells, they are most effective in patients whose tumors express these proteins. Enfortumab Vedotin targets Nectin-4, a protein highly expressed in bladder cancer, while Sacituzumab Govitecan targets Trop-2, which is found in many cancers. Doctors may perform biomarker testing to determine whether a patient’s tumor has enough of these proteins for ADC therapy to be effective.
ADCs may also be a good option for patients who cannot receive standard chemotherapy. Some individuals have kidney disease, making cisplatin-based chemotherapy unsafe, while others experience severe side effects that prevent them from continuing traditional treatments. For these patients, ADCs provide an alternative that may be both safer and more effective.
How ADCs Work in Bladder Cancer?
Bladder cancer cells often express specific surface proteins that can be targeted by ADCs. One of the most notable is nectin-4, which is highly expressed in many bladder tumors. When an ADC, such as Enfortumab vedotin, binds to nectin-4, the drug is internalized by the cancer cell. Once inside, the ADC releases its toxic payload, destroying the cell from within.

Another target, Trop-2, is also prevalent in bladder cancer. Sacituzumab govitecan, an ADC that targets Trop-2, has shown promise in treating patients who no longer respond to chemotherapy or immunotherapy.
Key ADCs in Bladder Cancer Treatment
Antibody-drug conjugates (ADCs) have become an important treatment option for advanced bladder cancer, offering a targeted approach that delivers chemotherapy directly to cancer cells while minimizing damage to healthy tissues. These drugs have shown significant success in patients whose cancer has progressed despite chemotherapy or immunotherapy.
Enfortumab Vedotin (Padcev) is one of the most well-established ADCs in bladder cancer treatment. It targets Nectin-4, a protein highly expressed in urothelial carcinoma, the most common type of bladder cancer. The FDA initially approved Enfortumab Vedotin in December 2019 for patients with advanced bladder cancer who had previously received chemotherapy and immunotherapy. In April 2023, the FDA granted full approval for its use in combination with pembrolizumab (Keytruda) as a first-line treatment for patients who cannot receive cisplatin-based chemotherapy. Clinical trials, including the EV-302 study, demonstrated that this combination significantly improved survival and response rates compared to standard chemotherapy.
Sacituzumab Govitecan (Trodelvy) is another FDA-approved ADC that targets Trop-2, a protein found on many bladder cancer cells. The FDA granted accelerated approval for this drug in April 2021 for patients with locally advanced or metastatic urothelial carcinoma who had previously been treated with platinum-based chemotherapy and immunotherapy. Clinical trials showed that Sacituzumab Govitecan improved progression-free survival and overall survival in patients whose disease continued to grow despite previous treatments.
Other ADCs, such as Disitamab Vedotin (RC48), are currently in clinical trials and may provide additional options for patients with bladder cancer, particularly those with HER2-positive tumors. As research continues, ADCs are being explored for earlier stages of bladder cancer, potentially offering new treatment strategies that could replace or enhance existing therapies.
How Different Types of Bladder Cancer Affect Treatment with Padcev/Enfortumab Vedotin?
Bladder cancer encompasses a variety of histological subtypes, which can significantly influence how the disease behaves and responds to treatment. One of the promising therapies for advanced bladder cancer is Enfortumab Vedotin (EV), an antibody-drug conjugate that specifically targets cancer cells. However, recent studies have highlighted that not all bladder cancer types respond equally to EV.
In the UNITE study, researchers examined the effectiveness of EV in 566 patients with advanced bladder cancer, categorizing them into three groups based on the histological characteristics of their tumors:
- Pure Urothelial Carcinoma (pUC) – the most common form of bladder cancer.
- Mixed Histology – tumors that contain both urothelial carcinoma and variant histologies.
- Pure Histological Variants (pHV) – tumors composed entirely of variant cancer cells.
Pure Urothelial Carcinoma (pUC)
This group showed the best response to EV treatment. Approximately 52% of patients experienced a significant shrinkage of their tumors. This suggests that traditional urothelial carcinoma cells are more susceptible to EV’s action, likely due to their expression of the target molecule, nectin-4, which EV binds to.
Mixed Histology
Patients with a combination of urothelial carcinoma and variant histologies had a 36% response rate, indicating a less favorable outcome than the pure urothelial carcinoma group. This suggests that the variant cell types within these tumors might reduce the drug’s effectiveness, possibly due to the presence of different molecular targets or resistance mechanisms.
Pure Histological Variants (pHV)
Patients whose tumors were entirely made up of variant cells showed 0% response to EV treatment. This underscores the difficulty in treating bladder cancers with variant histologies, which may lack the key target for EV or have alternate mechanisms of drug resistance. These tumors may require alternative treatment strategies, as EV appears ineffective in this context.
The UNITE study offers compelling insights into how bladder cancer’s histological makeup influences treatment outcomes. According to a study by Powles et al. published in the New England Journal of Medicine (2020), the difference in response rates among these subgroups can be attributed to the varying expression of nectin-4. Pure urothelial carcinoma, which has a high expression of nectin-4, is highly responsive to EV. In contrast, tumors with variant histologies may express lower levels of nectin-4 or none at all, making them less susceptible to the drug.
Which Types Responded Worst?
In addition to the general categories of bladder cancer, certain aggressive and variant histological types have shown particularly poor responses to Enfortumab Vedotin (EV). Among these, neuroendocrine (NE) bladder cancer showed no response at all to EV, with a 0% response rate. This type of cancer is known for its aggressive behavior and is notoriously difficult to treat with conventional therapies, likely due to its distinct molecular profile and lack of the nectin-4 target that EV requires to be effective.
Other aggressive forms, such as sarcomatoid and plasmacytoid bladder cancers, also showed a worse response compared to common urothelial carcinoma. Although these types had a slightly higher response rate than neuroendocrine cancer, they still lagged behind the more common forms of bladder cancer, underlining the challenge of treating these rare and aggressive variants with EV. Sacituzumab govitecan has demonstrated strong responses, particularly in those with heavily pre-treated metastatic bladder cancer.
What Makes Some Bladder Cancers Harder to Treat?
Doctors have identified several high-risk bladder cancer variants, including:
- Neuroendocrine (NE) bladder cancer – Extremely aggressive and does not respond well to standard treatments.
- Pure histological variants (pHV) – Bladder cancers that have completely different cell types than common urothelial carcinoma, often with poor treatment responses.
- Rare subtypes like sarcomatoid and plasmacytoid – Uncommon but can be difficult to treat.
Can We Improve Treatment for These Patients?
Since some bladder cancer types don’t respond well to EV alone, researchers are looking at combination therapies to improve survival:
Recent clinical trials show that combining Enfortumab Vedotin (EV) with Pembrolizumab (Keytruda) significantly improves outcomes in advanced bladder cancer. In the EV-302 trial, the EV + pembrolizumab combination doubled progression-free survival and increased overall survival rates compared to chemotherapy. Tumor shrinkage occurred in 67% of patients, with some achieving complete remission. This suggests that adding immunotherapy enhances EV’s effectiveness, especially for aggressive or resistant bladder cancers.If confirmed in further studies, this could replace chemotherapy as the first-line standard of care for bladder cancer.(New England Journal of Medicine, 2024)
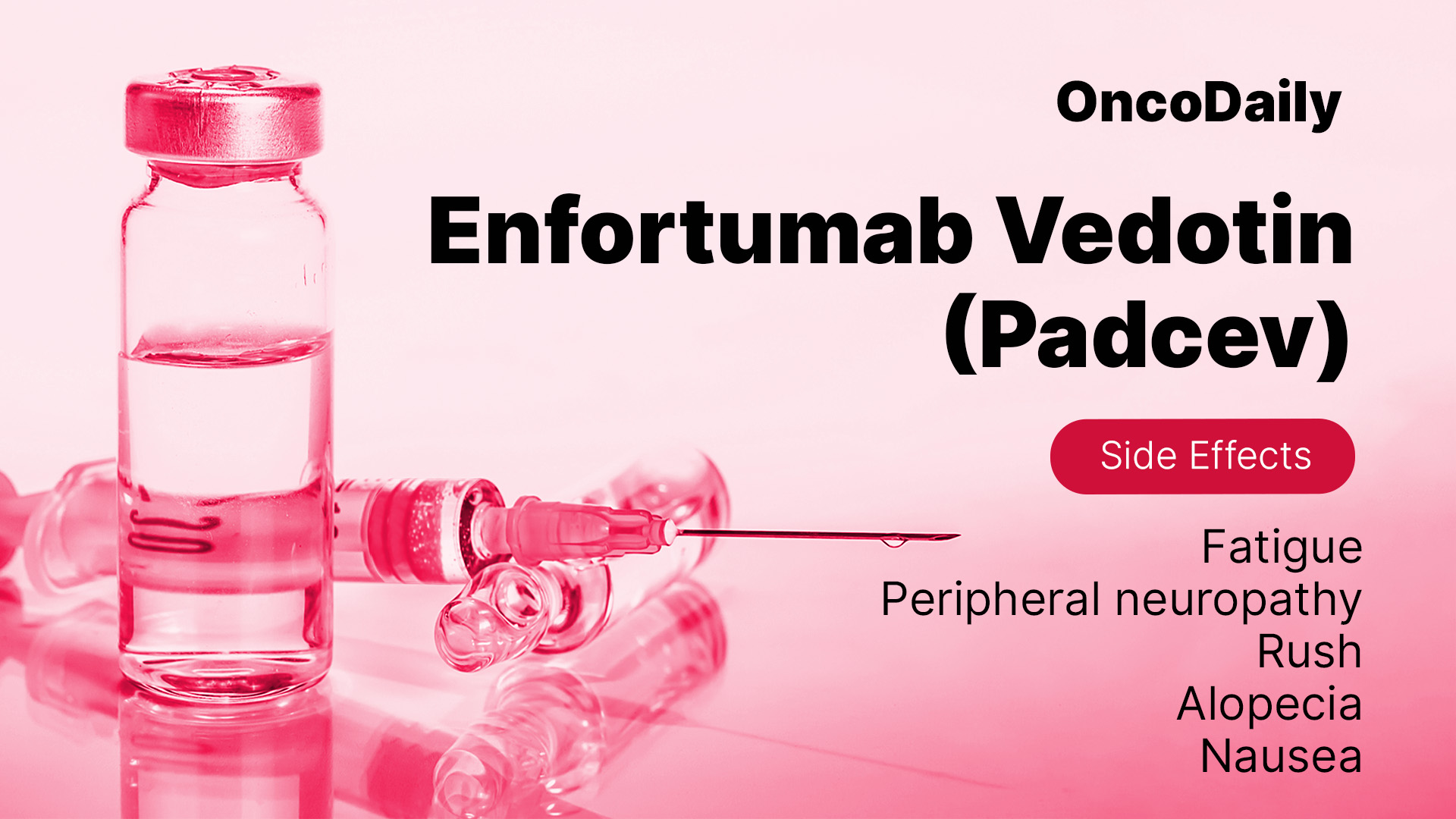
Common Side Effects of ADCs in Bladder Cancer
Most patients experience mild to moderate reactions, which can often be managed with supportive care. Understanding these side effects can help patients prepare for treatment and maintain their quality of life.
- Fatigue – Feeling tired or weak, common in cancer treatments.
- Nausea and loss of appetite – Can affect daily life but is often manageable.
- Peripheral neuropathy (nerve damage) – Tingling, numbness, or pain in hands and feet.
- Skin rash and irritation – Some patients experience redness, itching, or peeling skin.
- Diarrhea – Can lead to dehydration if severe.
ADCs in Early-Stage Bladder Cancer
For years, chemotherapy has been the standard treatment before surgery (neoadjuvant therapy) for muscle-invasive bladder cancer (MIBC). However, many patients cannot tolerate chemotherapy, leaving them with limited options. A promising new approach is being tested in the NCT05014139 clinical trial, which investigates whether Enfortumab Vedotin (EV) could be a more effective and safer alternative.
This study is exploring how EV, an antibody-drug conjugate (ADC), can shrink bladder tumors before surgery, potentially improving outcomes and offering hope to patients who cannot receive chemotherapy.
Now, researchers are testing whether EV can be effective in early-stage bladder cancer, before surgery.This phase II clinical trial is investigating EV as a neoadjuvant (pre-surgery) treatment for patients with muscle-invasive bladder cancer (MIBC) who are scheduled for bladder removal surgery (radical cystectomy).
Goal of the trial:
- To see if EV can shrink tumors before surgery.
- To determine if EV improves surgical outcomes compared to standard chemotherapy.
Why it matters:
- Many patients cannot receive cisplatin-based chemotherapy due to kidney issues or other health conditions.
- If EV proves effective, it could become a safer and more widely available option for bladder cancer patients.
The NCT05014139 trial represents a major step forward in bladder cancer treatment. If Enfortumab Vedotin proves to be safe and effective in early-stage disease, it could replace chemotherapy as the preferred pre-surgical treatment for many patients.
This trial is ongoing, but its results could change the way bladder cancer is treated in the future, offering a new path for patients seeking better, less toxic treatment options.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What are Antibody-Drug Conjugates (ADCs)
ADCs are a type of targeted cancer treatment that combines an antibody (which seeks out cancer cells) with a chemotherapy drug. This allows precise delivery of the drug directly into cancer cells, minimizing harm to healthy tissue.
How Antibody-Drug conjugates work in bladder cancer?
For bladder cancer, Enfortumab Vedotin (Padcev) targets Nectin-4, a protein found on most bladder cancer cells, while Sacituzumab Govitecan (Trodelvy) targets Trop-2, another cancer-associated protein
Who can receive ADC therapy for bladder cancer?
Enfortumab Vedotin is approved for patients with advanced or metastatic bladder cancer who have already been treated with chemotherapy or immunotherapy. Sacituzumab Govitecan is also approved for metastatic bladder cancer that has progressed after at least two prior treatments Clinical trials are now testing ADCs in earlier-stage bladder cancer, including before surgery (neoadjuvant therapy) or after surgery (adjuvant therapy).
How effective are ADCs in treating bladder cancer?
Enfortumab Vedotin has shown strong tumor shrinkage rates, even in patients whose cancer had resisted other treatments. Combining Enfortumab Vedotin with immunotherapy (like pembrolizumab) has doubled survival time in some studies.
What are the common side effects of ADCs?
Both ADCs have targeted effects but can still cause side effects, including:Fatigue, nausea, and diarrhea,peripheral neuropathy (nerve damage), causing tingling or numbness in hands and feet,skin reactions, including rashes or blisters,severe diarrhea (especially with Sacituzumab Govitecan)
Are ADCs replacing chemotherapy for bladder cancer?
Not yet, but ADCs are changing treatment strategies. Right now, they are mainly used after chemotherapy stops working, but trials are investigating whether they could replace or reduce the need for chemotherapy in early treatment stages.
How can I access ADC treatment for bladder cancer?
For approved treatments: Consult your oncologist to determine eligibility.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
