Immunotherapy for Brain Cancer: Types, Success Rate, Side Effects and More
Brain cancer, particularly aggressive forms like glioblastoma, remains one of the most challenging malignancies to treat. Traditional approaches, including surgery, radiation, and chemotherapy, often provide limited long-term success, leaving researchers in constant pursuit of more effective therapies. In recent years, immunotherapy has emerged as a promising avenue, harnessing the body’s own immune system to recognize and attack cancer cells.
This article explores the different types of immunotherapy currently used in brain cancer treatment, their success rates, potential side effects, and the latest advancements in research. While immunotherapy has revolutionized treatment for several cancers, its application in brain tumors presents unique challenges and opportunities. By understanding its mechanisms and effectiveness, we can gain insight into the future of brain cancer treatment and the hope it offers to patients.
What Is Immunotherapy for Brain Cancer?
Immunotherapy is a groundbreaking approach to cancer treatment that leverages the body’s own immune system to recognize and destroy cancer cells. Unlike traditional treatments such as surgery, chemotherapy, and radiation— which directly target tumors— immunotherapy enhances the immune system’s ability to detect and eliminate cancerous cells, offering a potentially more precise and lasting response.
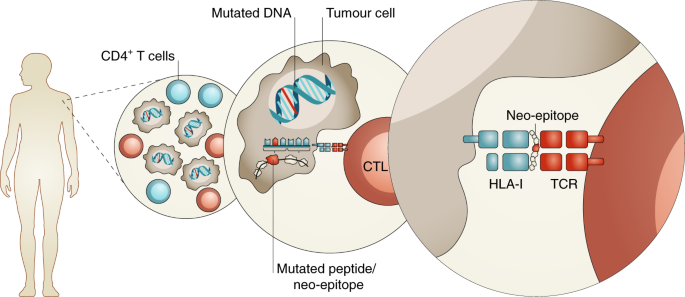
The immune system plays a crucial role in defending the body against harmful invaders, including viruses, bacteria, and abnormal cells. Under normal conditions, immune cells such as T cells and natural killer (NK) cells patrol the body, identifying and eliminating threats before they become harmful. However, cancer cells have developed sophisticated mechanisms to evade immune detection, often by disguising themselves as normal cells or suppressing immune responses. This ability allows tumors, including aggressive brain cancers like glioblastoma, to grow unchecked.
Immunotherapy aims to overcome these barriers by either boosting the immune system’s natural response or equipping it with targeted tools to recognize and attack cancer cells more effectively. Unlike chemotherapy, which destroys both healthy and cancerous cells, immunotherapy is designed to be more selective, reducing damage to normal tissues. This distinction is particularly important in brain cancer, where preserving healthy brain function is critical. While surgery and radiation physically remove or shrink tumors, immunotherapy has the potential to create long-term immune memory, helping the body prevent cancer recurrence.
What Are the Types of Immunotherapy for Brain Cancer?
Several types of immunotherapy are being explored and utilized in the treatment of brain cancer, particularly glioblastoma and other aggressive tumors. These include immune checkpoint inhibitors, which help restore the immune system’s ability to recognize and attack cancer cells, monoclonal antibodies, which target specific proteins on tumor cells, cancer vaccines, designed to stimulate the immune system to mount a response against brain cancer, and CAR T-cell therapy, a cutting-edge approach that involves genetically modifying a patient’s own immune cells to enhance their ability to fight the tumor.
Each of these therapies represents a unique strategy in the evolving landscape of brain cancer treatment, offering new possibilities where conventional methods have had limited success.
Checkpoint Inhibitors
Pembrolizumab (Keytruda) and nivolumab (Opdivo) are monoclonal antibodies that function as immune checkpoint inhibitors by targeting the programmed cell death protein 1 (PD-1) pathway. Under normal circumstances, the interaction between PD-1 receptors on T-cells and their ligands, PD-L1 or PD-L2, serves to regulate immune responses, preventing autoimmunity by inhibiting T-cell activity. However, many tumors, including glioblastomas, exploit this pathway by expressing PD-L1, thereby evading immune surveillance. By binding to PD-1 receptors, pembrolizumab and nivolumab block this interaction, effectively reactivating T-cells to recognize and attack cancer cells.
In glioblastoma treatment, the efficacy of these drugs has been the subject of various clinical trials. For instance, a phase III trial known as CheckMate-143 compared nivolumab to bevacizumab in patients with recurrent glioblastoma. The study found no significant improvement in overall survival (OS) for nivolumab, with a median OS of 9.8 months compared to 10.0 months for bevacizumab. Similarly, pembrolizumab has been evaluated in this context. A small, single-arm study demonstrated a 37.7% progression-free survival (PFS) rate at six months and a median OS of 13.1 months, suggesting some antitumor activity in patients with recurrent glioblastoma.
Despite these outcomes, ongoing research continues to explore the potential of PD-1 inhibitors in glioblastoma, particularly in combination with other therapies or in specific patient subgroups. For example, a phase II trial investigated the combination of tumor-treating fields (TTFields) with temozolomide and pembrolizumab in newly diagnosed glioblastoma patients, reporting improved survival outcomes.
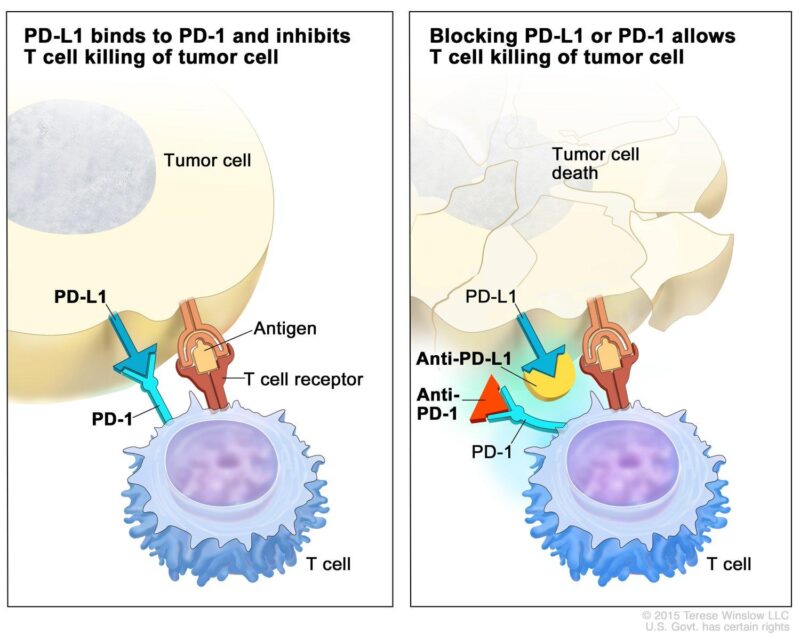
Monoclonal Antibodies
Bevacizumab (Avastin) is a humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF), a key protein responsible for promoting the growth of new blood vessels (angiogenesis) essential for tumor development and sustenance. By inhibiting VEGF, bevacizumab effectively restricts the blood supply to tumors, thereby hindering their growth and potential metastasis.
In the context of glioblastoma, a highly aggressive form of brain cancer, bevacizumab has been extensively studied. A notable study led by Mark R. Gilbert, M.D., published in The New England Journal of Medicine, evaluated the efficacy of bevacizumab in patients with newly diagnosed glioblastoma. The randomized trial compared standard therapy with and without the addition of bevacizumab. The results indicated that while progression-free survival was prolonged, there was no significant improvement in overall survival for patients receiving bevacizumab.
Cancer Vaccines
The development of vaccines to train the immune system to recognize and attack brain cancer cells has been an area of active research, focusing on both personalized vaccines and mRNA-based approaches.
Personalized cancer vaccines are designed to elicit an immune response tailored to the unique antigenic profile of an individual’s tumor. For glioblastoma, one such vaccine is DCVax-L, developed by Northwest Biotherapeutics. This vaccine utilizes a patient’s own dendritic cells, exposed to tumor lysate prepared from their resected tumor tissue, aiming to stimulate a targeted immune response against the tumor. In a Phase III clinical trial involving patients with newly diagnosed glioblastoma, DCVax-L demonstrated a median overall survival of 19.3 months, compared to 16.5 months in the external control group. Additionally, the 60-month survival rate was 13% for the DCVax-L group versus 5.7% for the control group. For recurrent glioblastoma, the mean overall survival was 13.2 months with DCVax-L treatment, compared to 7.8 months in the control group. Over 2,100 doses were administered, with only five serious adverse events reported.
Another personalized vaccine, ERC1671 (Gliovac), developed by Epitopoietic Research Corporation, combines tumor cells from the patient and multiple donors to present a broad array of tumor antigens to the immune system. In an initial compassionate use study involving nine patients with recurrent glioblastoma, 100% survived for six months compared to 33% in the control group, and 77% survived at ten months versus 10% in the control group. ERC1671 is currently undergoing Phase II trials in the United States.
The success of mRNA technology in COVID-19 vaccines has spurred interest in its application for cancer treatment. mRNA vaccines can be designed to encode tumor-specific antigens, prompting the immune system to target cancer cells expressing these antigens. Preliminary studies have shown promise in this area. For instance, a discussion on the Cancer Survivors Network highlighted ongoing clinical trials investigating mRNA vaccines for glioblastoma, with early results indicating potential efficacy. Moreover, pharmaceutical companies like BioNTech and Moderna, known for their COVID-19 mRNA vaccines, are actively developing mRNA-based cancer vaccines. A recent article in the Financial Times reported on a personalized mRNA vaccine trial for colon cancer, showing promising early results.
Recent advancements include a partnership between GlaxoSmithKline (GSK) and the University of Oxford to explore vaccines targeting pre-cancerous cells using neoantigen identification and mRNA technology. This collaboration aims to prevent the development of cancers by enabling the immune system to recognize and eliminate cells before they become malignant.
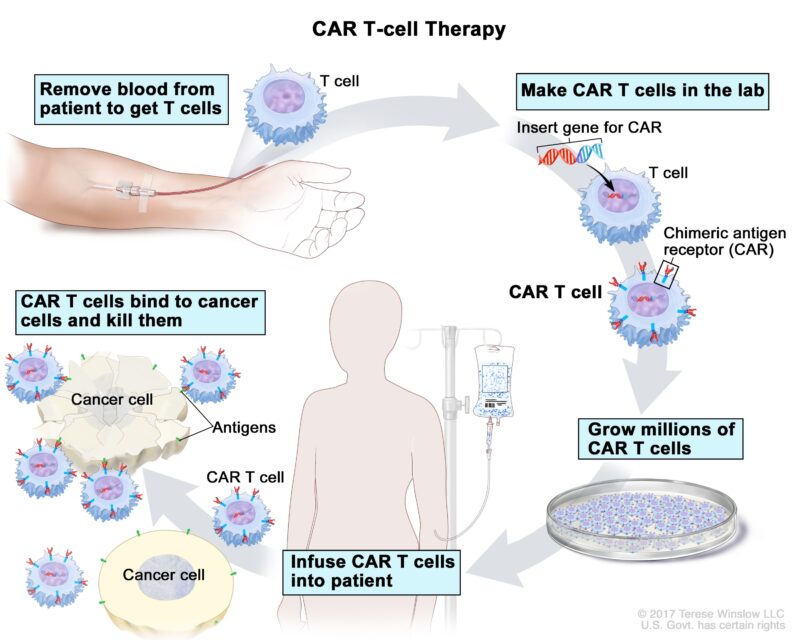
CAR T-Cell Therapy
Chimeric Antigen Receptor (CAR) T-cell therapy represents a novel approach in the treatment of glioblastoma, an aggressive form of brain cancer. This therapy involves engineering a patient’s own T-cells to express specific receptors that recognize and attack tumor cells.
The process begins by collecting T-cells from the patient’s blood. These cells are then genetically modified in the laboratory to express chimeric antigen receptors (CARs) that specifically target antigens present on glioblastoma cells. Once engineered, the CAR T-cells are expanded in number and infused back into the patient, where they seek out and destroy tumor cells expressing the target antigen. Several clinical trials have explored the efficacy of CAR T-cell therapy in glioblastoma patients:
- IL13Rα2-Targeted CAR T-Cells: A study led by Christine E. Brown, Ph.D., at City of Hope, investigated CAR T-cells targeting the IL13Rα2 antigen, which is overexpressed in glioblastoma. In this Phase I trial, 29 of 58 patients achieved stable disease for at least two months, with two partial responses and two complete responses observed.
- EGFRvIII-Directed CAR T-Cells: Research led by Donald M. O’Rourke, M.D., at the University of Pennsylvania, focused on CAR T-cells targeting the EGFRvIII mutation, common in glioblastoma. The Phase I trial demonstrated that a single dose of peripherally infused EGFRvIII-directed CAR T-cells could mediate antigen loss and induce adaptive resistance in patients with recurrent glioblastoma.
- GD2-Targeted CAR T-Cells: A study published in Nature reported on the use of GD2-targeted CAR T-cells in treating diffuse midline gliomas, a subset of glioblastomas. The trial demonstrated that GD2-CAR T-cell therapy induced clinical responses in patients with H3K27M-mutated diffuse midline gliomas, indicating potential efficacy in this hard-to-treat population.
How Effective Is Immunotherapy for Brain Cancer?
Glioblastoma (GBM) is renowned for its aggressive nature and poor prognosis. Traditional treatments, including surgery, chemotherapy, and radiotherapy, have achieved limited success in significantly extending patient survival. The median overall survival (OS) for GBM patients is approximately 12 to 18 months, with a five-year survival rate of about 5% . (American Cancer Society)
Surgical resection remains a cornerstone in GBM management. A study published in BMC Cancer by A. Almenawer et al. analyzed survival outcomes in GBM patients, revealing that patients who underwent surgical resection had a median OS of 9 months. Factors such as younger age, extent of resection, and adjuvant therapies were associated with improved survival .
Chemotherapy, particularly with temozolomide, has been integrated into standard GBM treatment protocols. However, its impact on long-term survival has been modest. The five-year survival rate remains low, underscoring the need for more effective therapeutic strategies . The advent of immunotherapy has introduced new avenues for GBM treatment, aiming to harness the body’s immune system to target tumor cells. Clinical trials have explored various immunotherapeutic approaches:
- Immune Checkpoint Inhibitors: A Phase III trial investigated the efficacy of nivolumab, an anti-PD-1 antibody, in recurrent GBM patients. The study, led by F. Mellinghoff et al. and published in The Oncologist, reported a median OS of 13.4 months for nivolumab-treated patients, which did not significantly differ from the control group .
- CAR T-Cell Therapy: Innovative approaches such as chimeric antigen receptor (CAR) T-cell therapy have shown promise in early-phase trials. A study by B. Choi et al., published in Nature, demonstrated that CAR T-cell therapy targeting specific GBM antigens resulted in tumor regression in a subset of patients. However, these findings are preliminary, and larger studies are necessary to validate efficacy .
- Cancer Vaccines: The development of vaccines aiming to elicit an immune response against GBM-specific antigens has been explored. A systematic review by A. Garsa et al., published in the International Journal of Molecular Sciences, analyzed various immunotherapeutic strategies, including vaccines, and found that 61% of the reviewed trials reported improvements in OS and/or progression-free survival (PFS) rates .
mdpi.com
What Are the Side Effects of Immunotherapy for Brain Cancer?
Immunotherapy for Brain Cancer can result in a range of side effects that vary in severity. While some side effects are common and generally manageable, others can be serious and require close monitoring.

Common Side Effects
Immunotherapy has revolutionized cancer treatment by harnessing the body’s immune system to target malignancies. However, its application can lead to various side effects, including fatigue, nausea, and skin rashes. Understanding the prevalence and management of these adverse events is crucial for optimizing. (According to a review published in Cancer Research by Naidoo et al.)
- Fatigue: Fatigue is a frequently reported side effect among patients undergoing immunotherapy. Fatigue occurs in approximately 16% to 24% of patients treated with immune checkpoint inhibitors.Management includes patient education, energy conservation strategies, and addressing contributing factors such as anemia or thyroid dysfunction. Exercise programs and psychosocial interventions have also shown benefit.
- Nausea: Gastrointestinal disturbances, such as nausea, are also common. Nausea affects about 12% to 20% of patients receiving these therapies.Antiemetic medications, dietary modifications, and adequate hydration are standard approaches to mitigate nausea. Identifying and managing other potential causes, such as concomitant medications or gastrointestinal conditions, is also important.
- Skin Rashes: Dermatologic adverse events, including skin rashes, are among the most prevalent side effects.Rash and pruritus (itchiness) occur in 20% to 40% of patients undergoing immune checkpoint blockade.Topical corticosteroids and oral antihistamines are commonly used to manage mild to moderate skin rashes. For severe cases, systemic corticosteroids may be necessary, and treatment interruption should be considered.
Serious Side Effects
Immunotherapy treatment can lead to severe side effects, including autoimmune reactions and central nervous system (CNS) inflammation, which require careful management.
Immune checkpoint inhibitors, such as pembrolizumab and nivolumab, work by blocking inhibitory pathways that suppress T-cell activity, thereby promoting an immune response against tumor cells. This mechanism, while effective against cancer, can also lead to immune-related adverse events (irAEs), in which the immune system attacks healthy tissue. Common ir
AEs include colitis, hepatitis, endocrinopathies, and dermatological reactions. In rare cases, patients may develop a myocarditis-myositis-myasthenia gravis overlap syndrome (IM3OS), characterized by simultaneous inflammation of the myocardium, skeletal muscle, and neuromuscular junction. A systematic review by Pathak et al. reported that approximately 60% of patients diagnosed with IM3OS experienced acute complications during hospitalization. Rapid recognition and management of irAEs are essential for patient safety. Initial treatment usually involves corticosteroids to suppress the immune response. In cases that do not respond to steroids, additional immunosuppressive agents such as infliximab or mycophenolic acid may be used. A case series by Siu et al. highlighted the importance of early intervention with immunosuppressants, noting that timely treatment resulted in resolution of irAEs in several patients.
CAR T-cell therapy, which involves engineering a patient’s own T cells to target cancer cells, has shown remarkable efficacy, particularly in hematological malignancies. However, it is associated with unique toxicities, particularly cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). ICANS manifests as encephalopathy, seizures, and in severe cases, cerebral edema. A study by Gust et al. Neurotoxicity following CAR T-cell therapy was observed to be associated with with elevated serum inflammatory markers associated with symptom severity. Management of CNS inflammation requires a multidisciplinary approach. Supportive care and close monitoring may be sufficient for mild neurotoxicity. Moderate to severe cases often require corticosteroid therapy to reduce inflammation. In some situations, additional interventions, such as antiepileptic drugs for seizure control, are warranted. A case report by Hill et al. detailed the occurrence of human herpesvirus 6 (HHV-6) encephalitis following CAR T-cell therapy, highlighting the need for vigilance in the diagnosis and management of infectious etiologies in immunocompromised patients.
Long-Term Side Effects
Patients may experience long-term side effects, particularly chronic immune system challenges and neurological effects.
Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, can lead to persistent immune-related adverse events (irAEs). A study led by Douglas Johnson, M.D., published in JAMA Oncology, found that over 40% of patients developed long-term irAEs, most of which were mild but persisted during the 1.5-year follow-up period. Neurological complications, though less common, have been reported. A study by John C. Probasco, M.D., in Brain Communications, reported that neurological irAEs occurred in 3.8% of patients treated with immune checkpoint inhibitors, with a mortality rate of 8%. Corticosteroid treatment led to neurological recovery in 74% of cases.
Long-term management of these side effects involves regular monitoring and prompt intervention. Corticosteroids are commonly used to manage irAEs, and early recognition is crucial to mitigate potential complications.
Immunotherapy for Stage 4 Brain Cancer
Treatment options for patients with stage 4 brain cancer, particularly glioblastoma, vary between monotherapy and combination therapy, each showing different levels of effectiveness. Monotherapy, which includes treatments like surgery, chemotherapy, radiation, or immunotherapy alone, can slow tumor progression but often has limited long-term success. In contrast, combination therapy—integrating multiple treatments such as immune checkpoint inhibitors with standard therapies—has shown potential to improve survival rates by targeting cancer through multiple mechanisms. While research continues to optimize these approaches, understanding the differences in their outcomes is crucial for selecting the most effective treatment strategy.
Combination Therapies
Combination therapies, integrating immunotherapy with traditional modalities like chemotherapy and radiation, have emerged as promising strategies in the treatment of advanced brain cancers, including glioblastoma. These approaches aim to enhance therapeutic efficacy by leveraging the synergistic effects of multiple treatments.
Combining immunotherapy with chemotherapy seeks to potentiate the immune response against tumor cells. Chemotherapeutic agents can induce immunogenic cell death, releasing tumor antigens that prime the immune system. When paired with immunotherapeutic agents, this can lead to a more robust anti-tumor response. However, clinical evidence supporting the efficacy of this combination in glioblastoma remains limited. A study by Reardon et al., published in the Journal for ImmunoTherapy of Cancer, evaluated the safety and efficacy of combining a dendritic cell vaccine with standard chemoradiotherapy in newly diagnosed glioblastoma patients. The results indicated that while the combination was safe, there was no significant improvement in overall survival compared to standard treatment alone.
Radiation therapy can modulate the tumor microenvironment, enhancing the visibility of cancer cells to the immune system. This has led to investigations into combining radiation with immunotherapy to improve patient outcomes. A review by Bernstein et al., published in the International Journal of Radiation Oncology, Biology, Physics, discussed the potential of this combination across various cancers, highlighting existing challenges and future directions. Ongoing clinical trials continue to explore various combination strategies to enhance the effectiveness of immunotherapy in glioblastoma treatment. For instance, a study by Zeng et al., published in Cell Research, reviewed the current state and challenges of immunotherapy for glioblastoma, emphasizing the need for novel combination approaches to overcome resistance mechanisms.
Monotherapy Options
Single-agent immunotherapy has been explored as a treatment modality for brain cancer patients, particularly those unable to tolerate combination therapies. However, its effectiveness has been limited.
- Immune Checkpoint Inhibitors: Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, have been evaluated as monotherapies in glioblastoma treatment. A study by Omuro et al., published in the Journal of Clinical Oncology, assessed nivolumab in patients with recurrent glioblastoma. The results indicated that nivolumab monotherapy did not significantly improve overall survival compared to the control group. Similarly, studies on pembrolizumab have shown limited efficacy as a single-agent treatment in glioblastoma patients.
- Oncolytic Virus Therapy: Oncolytic virus therapy involves using genetically engineered viruses to selectively infect and kill tumor cells. A study by Desjardins et al., published in the Journal of Clinical Oncology, investigated the use of the oncolytic poliovirus PVSRIPO in recurrent glioblastoma patients. The findings demonstrated a median overall survival of 12.5 months, suggesting potential benefits in a subset of patients.
- Dendritic Cell Vaccines: Dendritic cell vaccines aim to stimulate the immune system to recognize and attack tumor cells. A study by Liau et al., published in Clinical Cancer Research, evaluated a personalized dendritic cell vaccine in newly diagnosed glioblastoma patients. The results showed a median overall survival of 23.1 months, indicating a potential improvement over standard therapies.
What Research Is Being Done on Immunotherapy for Brain Cancer?
Recent advancements in immunotherapy for brain cancer have yielded promising developments, including novel drugs, combination therapies, and personalized medicine approaches.
- Chimeric Antigen Receptor (CAR) T-Cell Therapy: CAR T-cell therapy, which involves engineering a patient’s T-cells to target specific tumor antigens, has shown encouraging results. A clinical trial led by Dr. Crystal Mackall and Dr. Michelle Monje at Stanford Medicine treated children with diffuse midline gliomas using GD2-directed CAR T-cells. One participant experienced complete tumor regression, remaining cancer-free four years post-treatment.
- Dual-Action Immunotherapy: Researchers at the Walter and Eliza Hall Institute (WEHI) have developed a dual-action immunotherapy that not only targets and destroys aggressive brain cancer cells but also helps the immune system build a lasting defense against them.This approach utilizes CAR T-cell therapy to treat gliomas, an incurable brain cancer with limited treatment options.
- Combination Therapies: Combining immunotherapy with other treatments has been a focus of recent clinical trials. A Phase II study is evaluating the efficacy of combining pembrolizumab (an immune checkpoint inhibitor) with olaparib (a DNA damage response inhibitor) and temozolomide (a chemotherapy agent) in patients with recurrent glioblastoma. This trial aims to determine if the combination can enhance treatment effectiveness compared to standard therapies.
- Personalized Vaccines: The Epitopoietic Research Corporation (ERC) has developed ERC1671 (Gliovac), an immunotherapy vaccine designed to train the body’s immune system to attack glioblastoma using cells from the patient’s tumor and cells from three other donors. In initial studies, patients treated with ERC1671 showed improved survival rates compared to control groups.
These advancements underscore the potential of immunotherapy to improve outcomes for brain cancer patients. Ongoing research and clinical trials continue to explore and refine these innovative approaches.
How Is Immunotherapy Administered?
Immunotherapy for brain cancer is primarily administered through intravenous (IV) infusions, allowing the medication to circulate through the bloodstream and reach the tumor. Depending on the specific drug and treatment plan, patients typically receive infusions every two to four weeks in an outpatient setting. Each session can last 30 minutes to several hours, depending on the drug’s infusion rate and potential pre-medications needed to manage side effects.
The overall duration of immunotherapy varies based on treatment response and tolerance. Some patients remain on therapy for six months to two years, while others may continue indefinitely if their cancer remains stable and side effects are manageable. Physicians monitor treatment effectiveness through imaging scans and blood tests, adjusting schedules if needed. Understanding the treatment process helps patients prepare for the time commitment and potential effects of ongoing therapy.
What Should Patients Expect During Treatment?
The immunotherapy journey begins with an initial consultation, where the oncologist evaluates the patient’s medical history, tumor characteristics, and overall health to determine if they are a suitable candidate. Once a treatment plan is established, patients undergo baseline tests, including bloodwork and imaging scans, to track their progress over time.
During treatment sessions, which are often administered through intravenous (IV) infusions, patients are monitored for immediate reactions. Side effects such as fatigue, nausea, or skin rashes are assessed, and supportive care is provided as needed. Throughout the treatment course, oncologists schedule regular follow-ups—typically every few weeks—to review imaging results and blood tests. If the tumor responds well and side effects remain manageable, therapy continues as planned. However, if severe side effects or lack of response occur, adjustments may include dose modifications, temporary breaks, or switching to alternative treatments.
Even after completing immunotherapy, patients remain under long-term follow-up to monitor for recurrence and manage any lingering immune-related effects. This comprehensive approach ensures that treatment remains both effective and tolerable for each individual patient.
How Long Does It Take to See Results from Immunotherapy?
The timeline for seeing results from immunotherapy in brain cancer varies significantly among patients. Some may notice improvements within a few weeks, while others may need several months before significant changes appear on imaging scans. Unlike chemotherapy, which often produces a rapid tumor-shrinking effect, immunotherapy works by stimulating the immune system, which can take longer to mount a strong response.
Factors influencing the speed of response include the stage of cancer, with early-stage tumors generally responding better than advanced, treatment-resistant ones. The type of immunotherapy also plays a role—checkpoint inhibitors, for example, may take longer to show effects compared to CAR T-cell therapy, which can sometimes produce quicker but more intense responses. Additionally, a patient’s immune system strength and genetic makeup can impact how quickly and effectively their body reacts to treatment.
Regular imaging scans and biomarker tests help oncologists assess whether the treatment is working. If there’s no improvement after several months, adjustments may be made, including combining therapies or exploring alternative options.
What Are the Costs of Immunotherapy for Brain Cancer?
Immunotherapy for brain cancer can be expensive, with costs varying based on the type of treatment. Checkpoint inhibitors like pembrolizumab (Keytruda) and nivolumab (Opdivo) can cost $10,000 to $15,000 per dose, while CAR T-cell therapy often exceeds $400,000 per treatment. Personalized vaccines and oncolytic virus therapies also come with high price tags, especially since many are still in clinical trial phases.
Insurance coverage depends on the patient’s provider and policy. Some immunotherapies, especially FDA-approved drugs, are covered under Medicare, Medicaid, and private insurance, but out-of-pocket expenses can still be significant. Patients may need pre-authorization or appeals if coverage is initially denied.
For those struggling with costs, financial assistance programs are available. Organizations like the American Cancer Society, the Patient Advocate Foundation, and pharmaceutical company assistance programs offer grants, co-pay assistance, or free medication for eligible patients. Clinical trials also provide access to experimental therapies at reduced or no cost, making them an important option for some patients. Exploring these resources early can help ease the financial burden of treatment.
Can All Brain Cancer Patients Receive Immunotherapy?
Not all brain cancer patients are eligible for immunotherapy, as eligibility depends on several key factors, including biomarkers, tumor characteristics, and overall health status. One of the most important biomarkers used to assess suitability is PD-L1 expression. Tumors that express high levels of PD-L1 are more likely to respond to immune checkpoint inhibitors like pembrolizumab or nivolumab, as these drugs help restore the immune system’s ability to recognize and attack cancer cells. However, glioblastomas and other brain tumors often have low PD-L1 expression, making immunotherapy less effective in many cases.
Additionally, tumor mutation burden (TMB) and the presence of specific genetic alterations, such as EGFR mutations or IDH mutations, can influence treatment decisions. Some tumors with microsatellite instability (MSI-high) or mismatch repair deficiency (dMMR) respond better to immunotherapy, as they tend to generate more neoantigens that trigger an immune response. CAR T-cell therapy eligibility also depends on whether the tumor expresses certain targetable proteins, such as IL13Rα2 or EGFRvIII.
Beyond genetic factors, oncologists also consider the patient’s overall health, immune function, and prior treatments. Patients with weakened immune systems or severe autoimmune diseases may not tolerate immunotherapy well, as it can overstimulate the immune system and cause harmful side effects. Clinical trials also offer access to experimental immunotherapies for patients who may not meet standard eligibility criteria.
How Can Patients Support Their Immune System During Treatment?
Supporting the immune system during brain cancer treatment involves a holistic approach encompassing diet, exercise, sleep, stress management, and cautious use of supplements.
- Diet: A nutrient-rich diet is foundational for immune support. Incorporating a variety of fruits, vegetables, whole grains, lean proteins, and healthy fats provides essential vitamins and minerals. For instance, the Mediterranean diet, rich in vegetables, pulses, healthy fats, and olive oil, has been associated with a reduced risk of certain cancers. Additionally, citrus fruits high in vitamin C can enhance immune function by increasing white blood cell production and reducing inflammation.
- Exercise: Regular physical activity is beneficial for maintaining immune health. Engaging in moderate exercise, such as walking or cycling, can improve circulation and promote the efficient functioning of immune cells. It’s advisable to tailor exercise routines to individual energy levels and treatment plans, ensuring activities are safe and manageable.
- Sleep: Adequate sleep is crucial for immune system regeneration. Aim for 7-9 hours of quality sleep per night to allow the body to repair and strengthen its defenses. Establishing a consistent sleep schedule and creating a restful environment can aid in achieving restorative sleep.
- Stress Management: Chronic stress can suppress immune function. Incorporating stress-reduction techniques such as meditation, deep breathing exercises, or yoga can help manage stress levels, thereby supporting immune health.
- Supplements: While certain supplements may offer immune support, it’s essential to approach them with caution. For example, vitamin D is vital for immune function, and supplementation may be necessary, especially in cases of deficiency. However, high doses of some supplements, such as antioxidants, could interfere with cancer treatments. Therefore, it’s imperative to consult with a healthcare provider before starting any supplement regimen.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is immunotherapy for brain cancer?
Immunotherapy is a treatment that enhances the body’s immune system to recognize and attack brain cancer cells, particularly in aggressive tumors like glioblastoma.
How does immunotherapy differ from chemotherapy and radiation
Unlike chemotherapy and radiation, which directly kill cancer cells, immunotherapy strengthens the immune system’s ability to fight cancer, offering a more targeted and potentially long-lasting response.
What are the different types of immunotherapy used for brain cancer?
Key types include checkpoint inhibitors, CAR T-cell therapy, monoclonal antibodies, cancer vaccines, and oncolytic virus therapy, each working to boost immune activity against tumors.
Does immunotherapy work for glioblastoma?
While immunotherapy has shown promise in some cancers, glioblastoma presents challenges due to its highly immunosuppressive environment. Clinical trials continue to explore its effectiveness.
What is the success rate of immunotherapy for brain cancer?
Success rates vary depending on the type of immunotherapy and patient response. Some treatments have improved survival in clinical trials, but widespread success is still under study.
What are the side effects of immunotherapy for brain cancer?
Common side effects include fatigue, nausea, rashes, and inflammation of organs like the lungs or liver. Severe cases may lead to neurological or autoimmune complications.
Are there clinical trials for immunotherapy in brain cancer?
Yes, many clinical trials are testing new immunotherapies, including personalized vaccines, combination therapies, and CAR T-cell approaches to improve treatment outcomes.
Can immunotherapy be used alongside other treatments?
Yes, researchers are exploring combinations of immunotherapy with chemotherapy, radiation, and targeted therapies to enhance effectiveness and prolong survival
How long does it take for immunotherapy to show results?
Responses vary, with some patients noticing effects within weeks, while others may take months. Regular monitoring through imaging scans helps assess treatment progress.
Is immunotherapy covered by insurance?
Some FDA-approved immunotherapies are covered by insurance, but experimental treatments in clinical trials may require financial assistance programs or self-payment.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
