
Talha Badar: Bispecific and immune conjugates in the management of AML
Talha Badar, Hematology/Oncology specialist at Mayo Clinic, shared a thread on X:
“Brief discussion on bispecific and immune conjugates (ADCs) in the management of AML
Limitations of immune-based therapy in AML:
(a) immunosuppressive microenvironment in AML
(b) off-target toxicity
(c) potent target that involves in leukemogenesis, not readily expressed in HSC is lacking.
Target antigen:
Unlike B-cell malignancies, ideal target for AML is yet to be identified.
Ideal target:
- Readily expressed on AML cells
- Ideally, not be expressed on healthy tissue to prevent off-target toxicity
- Ideal target should also be involved in leukemogenesis
Target antigen evaluated in ADCs and bispecific – CD33, CD123, CD135/FLT3, CD70, TIM-3
Gemtuzumab ozogamicin (GO) only immunotherapeutic agent approved for AML.
Most active in combination with intensive chemotherapy in favorable risk AML
Efficacy as monotherapy in RR AML is limited
Cusatuzumab is a high-affinity, anti-CD70 mAb was evaluated in PI/II study in combination with AZA. Most common toxicity: myelosuppression and infections. ORR 50%, CR 36.8%.
Cusatuzumab in being evaluated in combination with AZA+Venetoclax
Flotetuzumab bispecific antibody-based molecule to CD3ε and CD123 engineered in a DART format.
Evaluated in P1/Ib study in RR AML ORR 30%, CRh 26.7% (Higher responses were observed in TP53m AML) mOS 10.2 mo.
Most common AE’s G1-II CRS. Did not lead to significant myelosuppression.
Lintuzumab radiolabeled anti-CD33 antibody, composed of an alpha emitting isotope, Ac225, conjugated with the humanized anti-CD33 antibody lintuzumab.
Unlike other radioactive cytotoxic therapies, radioactive precautions are not required allowing its administration in inpatient as well as outpatient setting In combination with intensive chemo (CLAG-M), ORR 67%. CR/CRi 53%. 2 yrs OS 53%.
Myelosuppression was frequent with delayed neutrophil and platelet recovery (33 to 55 days) Lintuzumab plus venetoclax in RR AML is ongoing.
Talacotuzumab, a humanized anti-CD123 mAb in combination with decitabine in elderly AML, evaluated in randomized fashion.
CR 15%, mOS 5.36 mo (Study)
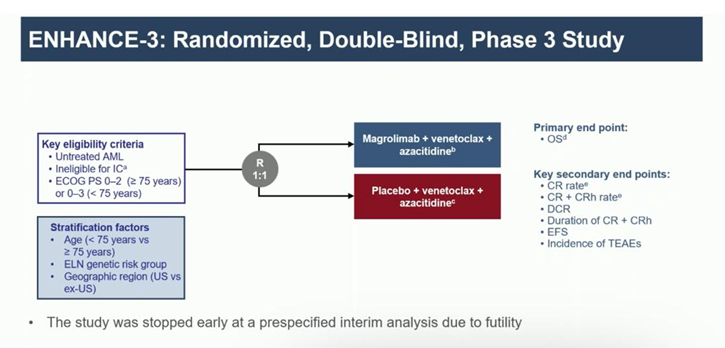
Magrolimab, humanized mAb targets CD47. After initial promising data, evaluated in a randomized PIII study vs placebo in combination with AZA + Ven. PE: OS Higher rates of death in the intervention arm due to infections. PE not met. (data presented by Dr. Daver at EHA 2024)
Sabatolimab is a humanized mAb (hIgG4, S228P) directed against human T-cell immunoglobulin domain and mucin domain-3 (TIM-3) evaluated in HR-MDS/AML in a phase 1b study.
34 evaluable pts with ND-AML, ORR 41.2% (8 CR, 3 CRi), 6 mo DOR 85%. 12 mo PFS 44%.
Common AE’s myelosuppression, infection, immune mediated AEs (arthritis, rash, hypothyroidism)
Conclusion:
Bispecific/ADCs fails to show benefit in AML unlike B -cell Malignancies.
It relies on T-cell fitness, most of patientss treated in trial had high disease burden with poor disease phenotype.”
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
