Amar Kelkar, Physician at the Dana-Farber Cancer Institute and an Instructor in Medicine at Harvard Medical School, shared on X/Twitter:
“Today in Annals of Internal Medicine: We show Axi-cel and Liso-cel are NOT COST-EFFECTIVE in our decision analysis of second-line CAR-T therapies for Diffuse Large B-Cell Lymphoma, and calculate the economic impact to be >$6.8 billion over 5 years! See here.
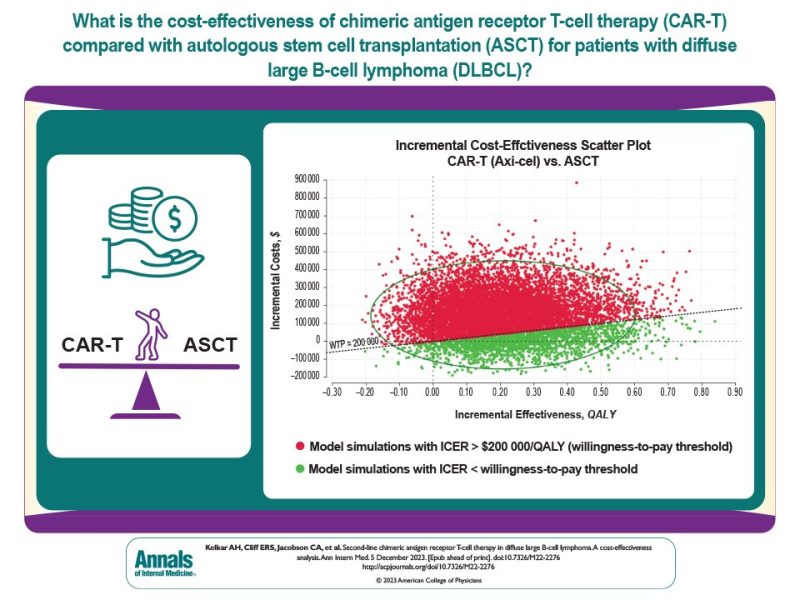
Only 20-30% with relapsed/refractory DLBCL achieve durable remissions with standard-of-care second-line salvage chemoimmunotherapy with autologous stem cell transplantation (ASCT). Novel CAR-T therapies improve survival over ASCT but cost >$400k/infusion!

We used a microsimulation model to evaluate if axi-cel and liso-cel are cost-effective for DLBCL second-line treatment compared to ASCT. This allowed us to model real-world situations to predict lifetime costs and lifetime effectiveness for 10,000 simulated patients.
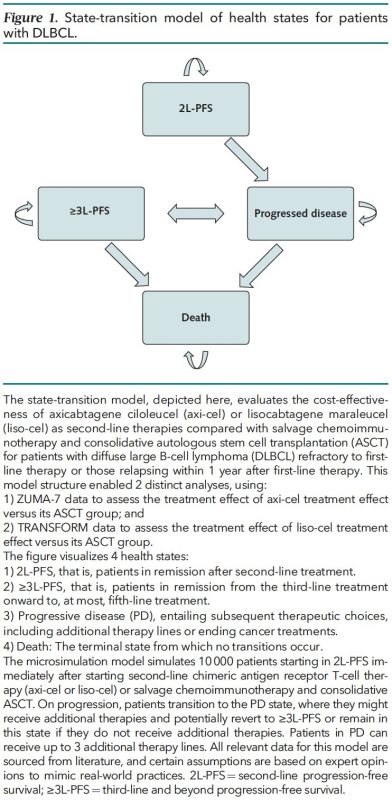
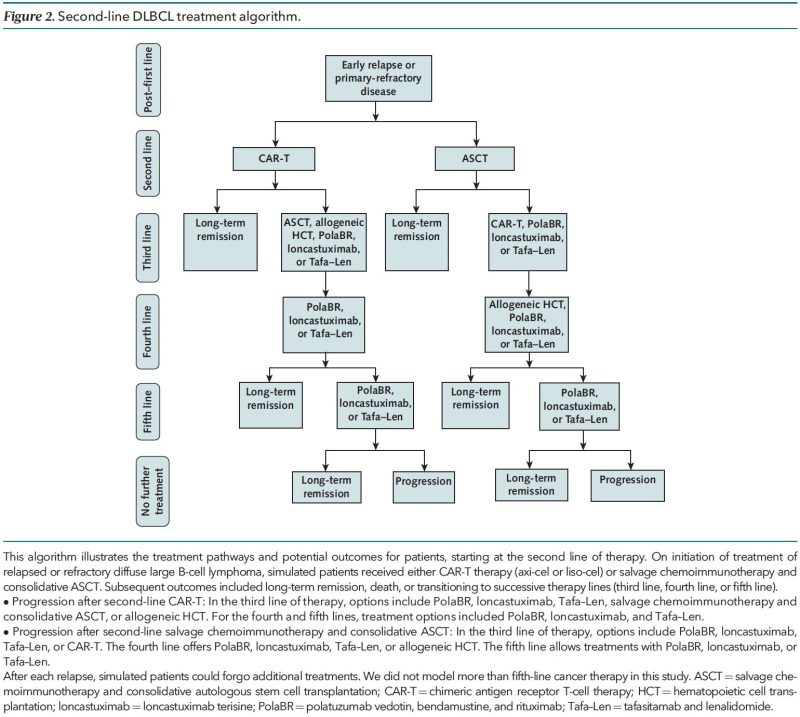
We modeled:
- 2nd to 5th line therapies for DLBCL using real-world practice patterns
- Costs: US
- Effectiveness: Measured in quality-adjusted life-years
- Discounting Rate: 3%/year (present costs and effectiveness more valuable than theoretical future costs and effectiveness)
There is no prescribed value cutoff in the US, so we modeled for a very high willingness-to-pay (WTP) threshold of $200,000/QALY to reflect decision-makers’ willingness to spend more on oncology therapies. Countries that utilize WTP use a lower number (~$50,000-100,000/QALY).
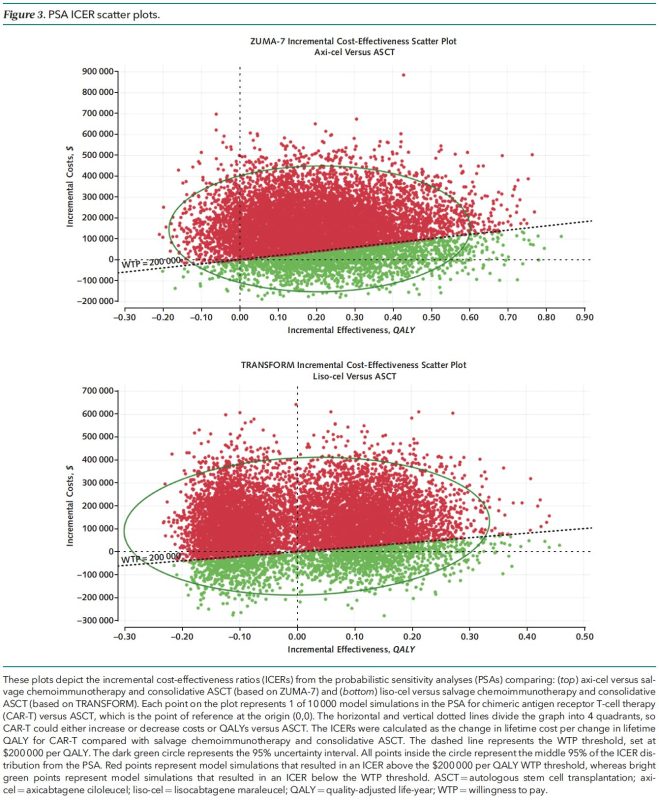
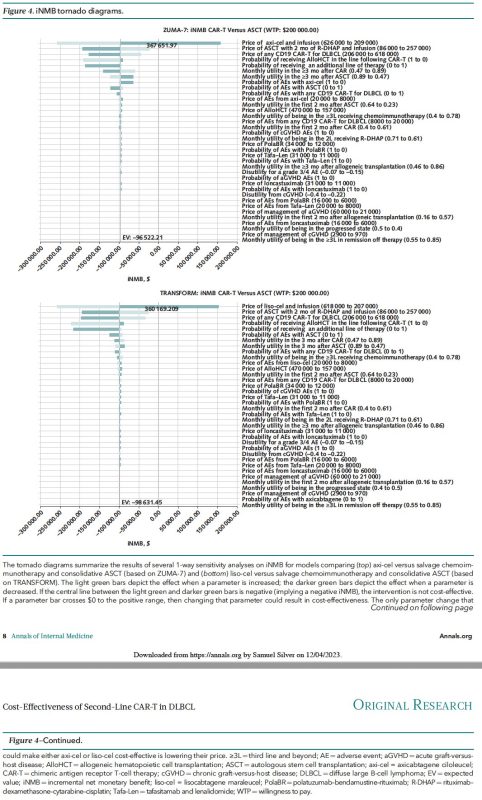
Sensitivity analyses included probabilistic sensitivity analysis (10,000 simulations of the 10,000-patient model) and assessment of which parameters (prices, adverse event frequencies, etc.) changed model outcomes from “cost-effective” to “not cost-effective” or visa versa.
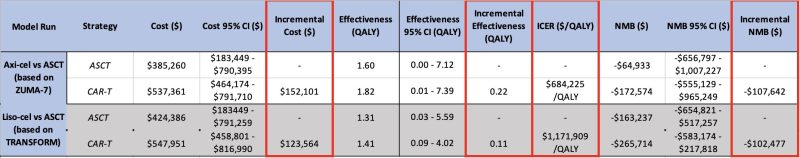
The model confirmed improved survival and quality of life with axi-cel and with liso-cel compared to ASCT! Median overall survival increased by 4 months for axi-cel and 1 month for liso-cel.
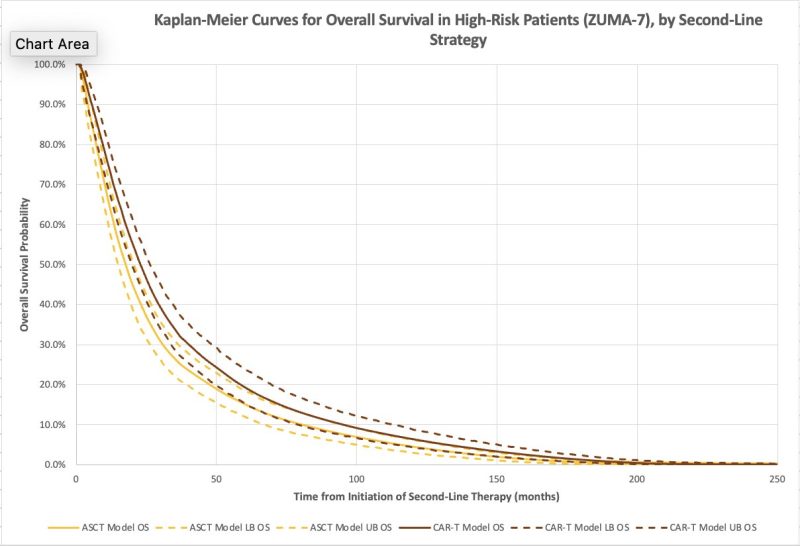
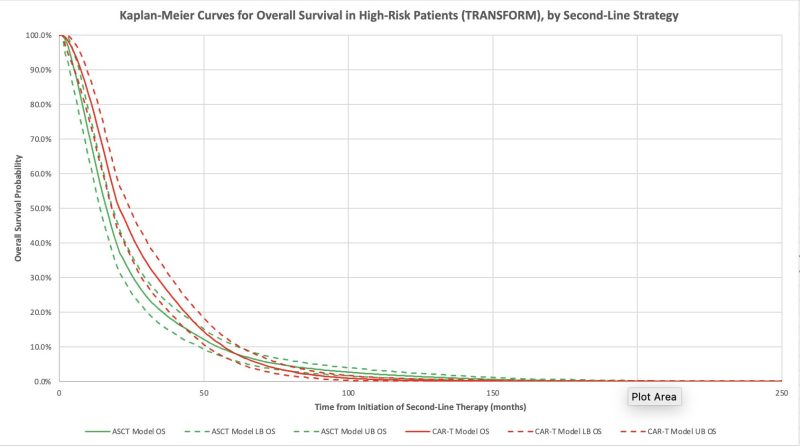
a
a
a
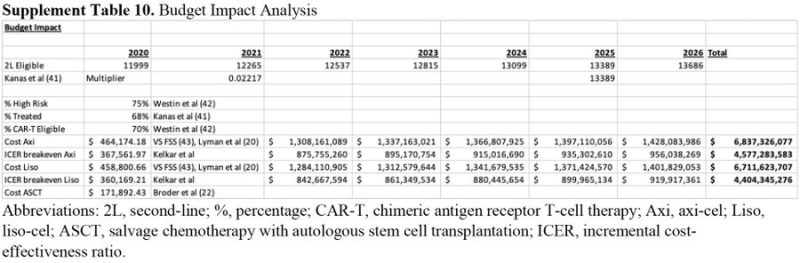
Budget Impact Analysis: Treatment of high-risk patients with DLBCL with 2nd-line CAR-T (over ASCT) adds $6.7-6.8 billion to US healthcare spending over 5 yrs! With >1000 active CAR-T trials under study, the impact of broad adoption of these therapies is astronomical!
A
a
While offering clinical benefit, axi-cel and liso-cel are NOT COST-EFFECTIVE for second-line DLBCL treatment at current prices. Price reduction is crucial for broader adoption! The exclusion of cell therapies from Medicare drug price negotiations must be reconsidered.
In summary, CAR-T, a therapy that costs almost 3x ASCT, but only improves survival by 1-4 months, is NOT COST-EFFECTIVE.
This one has been a long time coming! Thanks to all my coauthors and mentors for helping bring this together. We hope putting out independent cost-effectiveness analyses like this will encourage others to join.”
