
Svetlana Nikic: Unlocking Access to Innovative Diagnostics in Europe
Svetlana Nikic, Founder of Precision Oncology Consulting, shared on LinkedIn:
“Molecular diagnostics are central to precision oncology, enabling targeted therapies by identifying specific genomic alterations in tumors. These tests also identify prognostic biomarkers, predicting long-term patient outcomes, and can refine initial diagnoses, improving diagnostic accuracy.
Numerous cancer-related biomarkers, such as HER2, NTRK, BRAF, and ALK, are currently used in routine cancer care, with many more cancer-specific and pan-cancer biomarkers emerging. Studies have demonstrated that patients receiving biomarker-matched therapies experience better outcomes compared to those treated with traditional cytotoxic chemotherapies.
However, introducing a novel molecular diagnostic test (without an existing comparable and reimbursed solution) into the European healthcare system is a complex process. As highlighted recently by Horgan et al. [3], the journey from assay development and validation to routine clinical use can take over a decade. Their work details the challenges faced by OncoTypeDX, a prognostic and predictive tool that, among other benefits, identifies a subgroup of breast cancer patients for whom adjuvant chemotherapy offers no benefit, significantly impacting patient quality of life. Figure 1 summarizes the lengthy process and key milestones in OncoTypeDX’s implementation.
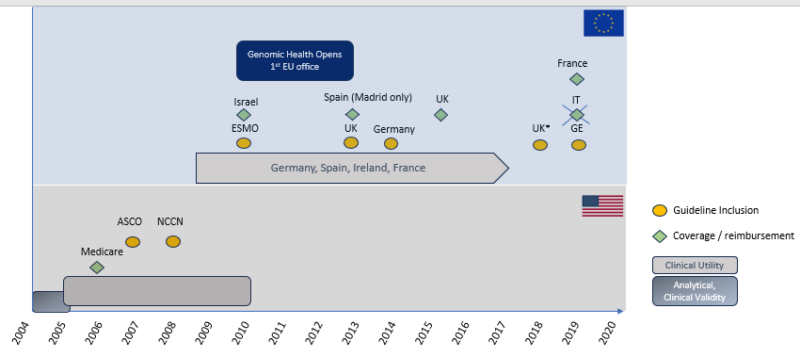
Following analytical and clinical validation, as well as assessments of clinical and economic utility, manufacturers must engage with local Health Technology Assessment (HTA) bodies and payers. HTA bodies are critical in evaluating the clinical effectiveness and cost-effectiveness of new health technologies, including biomarker tests, to inform reimbursement decisions across the EU.
Examples of HTA bodies include the National Institute for Health and Care Excellence (NICE) in the UK, Zorginstituut Nederland in the Netherlands, KCE (Belgian Health Care Knowledge Centre) in Belgium, and AETS (Agencia de Evaluación de Tecnologías Sanitarias) in Spain. After evaluating a test’s clinical effectiveness and cost-effectiveness, the HTA results are presented to policymakers, who then decide on adoption, funding, and regulation within their respective healthcare systems.
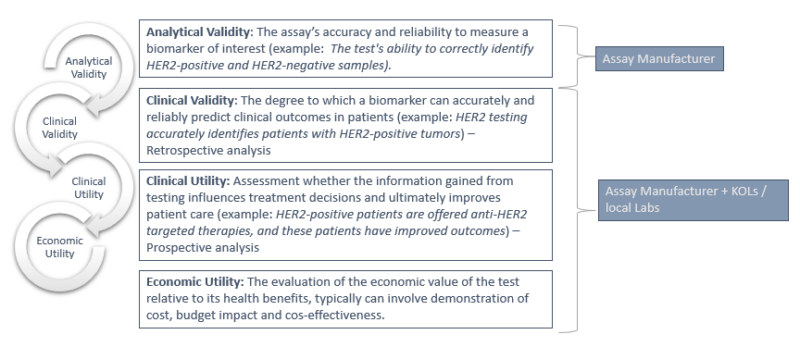
Early engagement with Key Opinion Leaders (KOLs) – clinicians who can support manufacturers through evidence generation, impact studies, and contribution to European guideline development – is crucial. Ideally, incorporating a novel approach into international and national guidelines should precede or coincide with the presentation of HTA results to policymakers. However, integrating new methods into European clinical guidelines is generally more challenging than in the US with, for example, the NCCN Guidelines. In Europe, a clinical utility study, ideally conducted by a pan-European group of clinicians and published in a peer-reviewed journal, is typically required.
When clinical utility is uncertain, KOLs, along with manufacturers’ Governmental Affairs and Market Access teams, may collaborate with ministries of health to design implementation studies. While some countries rely on HTA evaluations for coverage decisions, others prioritize implementation studies, national programs, and legislation. Examples include the UK’s NHS Genomic Medicine Service program and France’s Plan Cancer 2014-2019, both of which have positively influenced reimbursement landscapes.
With this short article, I hope I managed to briefly represent the diverse reimbursement pathways that we have across the EU which necessitate robust clinical evidence generation, thorough economic analyses, and proactive stakeholder engagement from manufacturers of novel diagnostic tools. Development of a comprehensive European Reimbursement Roadmap is highly recommended, including evidence generation and KOL engagement strategies, along with early dialogue with local HTA bodies and payers. All of these, strategically planned activities can lead to favorable reimbursement, ultimately ensuring patient access to innovative diagnostic solutions.”
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
