
Charles Jiang: Medicare Part D requires prior authorization for Ondansetron in 80% of plans
Charles Jiang, Assistant Professor of Internal Medicine at UT Southwestern Medical Center and Genitourinary medical oncology, shared an article on X:
“As an oncologist, I discovered something shocking:
Medicare Part D requires prior authorization for ondansetron(Zofran) – a basic anti-nausea med – in 80% of plans!
Here’s why this matters…”
“Found this shocking fact (80% PA rate) by accident while digging through data. Had to double-check with our pharmacists because I couldn’t believe it.
This is a GENERIC drug that’s so affordable, WHO recommends it for low-resource countries!”
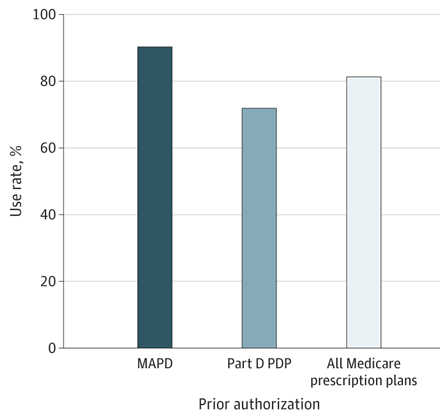
“Reality check: My cancer patients are either:
– Waiting days for approval.
– Paying cash to avoid delays
– Getting hit with nausea & maybe ending up in ER.
All to get a cheap, proven drug that prevents vomiting during chemo.”
“Some say ‘What’s the big deal if all PAs get approved?’
But that’s exactly the point – why have paperwork that delays care if we’re approving everything anyway?
Just adding needless barriers.”
“My theory: These PA rules were set decades ago when ondansetron was expensive.
Now it’s cheap and generic, but no one bothered to remove the red tape.
Healthcare bureaucracy on autopilot while patients suffer.”
“Think about it: Every delay means:
– Patients suffering needlessly.
– More ER visits for nausea.
– Extra costs for everyone.
– Hours of paperwork for my staff.”
“Bottom line: Sometimes the best cost savings in healthcare isn’t adding more paperwork – it’s removing barriers to basic, affordable care.
Time to rethink these outdated PA requirements! ”
Costs and Access Barriers to Ondansetron in the US.
Authors: Changchuan Jiang, et al.

-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
