
Nima Sharifi: An inherited physiologic driver of prostate cancer mortality and potential treatment strategies
Nima Sharifi, Scientific Director of Desai Sethi Urology Institute, shared a post on X about a recent paper published in Journal of Clinical Investigation.
Authors: Nima Sharifi, Robert Diaz, Hui-Ming Lin, Evan Roberts, Lisa Horvath, Andrew Martin, Martin Stockler, Sonia Yip, Vinod Subhash, Neil Portman, Ian Davis, Christopher Sweeney

“Thrilled that our story on HSD3B1 in ENZAMET is out in Journal of Clinical Investigation, ASCI!
This is a new level of evidence for an inherited physiologic driver of prostate cancer mortality and potential treatment strategies!
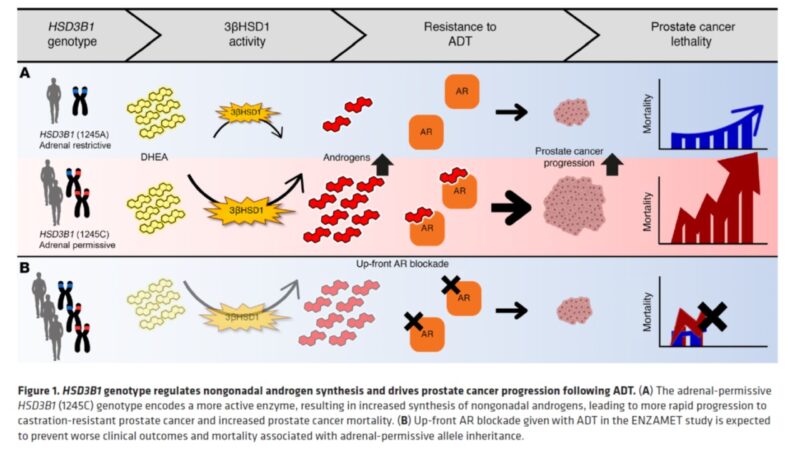
Worth emphasizing again that the missense-encoding HSD3B1 adrenal-permissive allele effectively increases activity for the first and rate-limiting step for pca to make DHT from non-gonadal precursors. It is inherited in 50% of all men! (link)
Inheritance of the HSD3B1 adrenal-permissive allele is associated with more rapid CRPC and shorter survival after ADT in men with low volume (LV) mHSPC b/c it allows tumors to use adrenal androgens more efficiently. (link)
Also – recent data from Rana McKay, Tyler Seibert and the Million Veterans Program (MVP) show HSD3B1 homozygosity linked to prostate cancer mortality in study of over 5000 men. That’s more common than germline BRCA mutations! (link)
But is there anything that can be done about it, particularly in men with LV mHSPC? What if we block the effect of non-gonadal androgens upfront (with enzalutamide or NSAA)? ENZAMET seemed perfect to answer this question! (link)
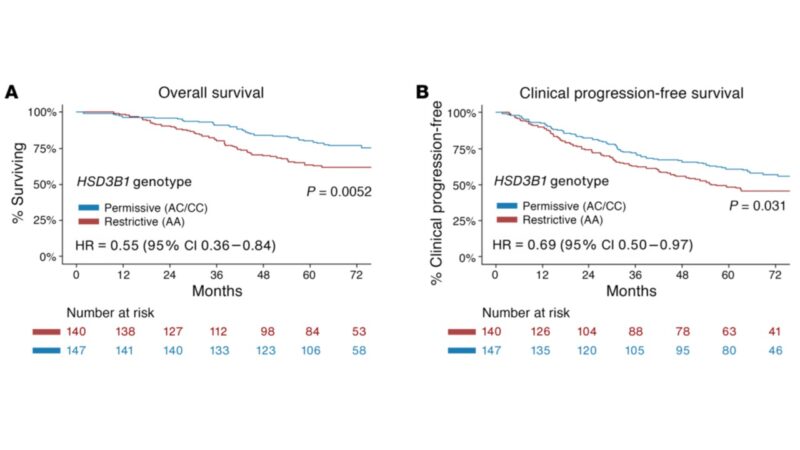
Here is OS for men with LV mHSPC tx with ADT+AR blocker only (no dcx). Men with adrenal-permissive HSD3B1 inheritance who have low survival in all the above studies actually have high survival compared with men who do not have adrenal-permissive inheritance! Similar with PFS!
Does HSD3B1 provide independent information? Or is it captured with other clinical variables (path, etc.)? MVP suggests that it is independent. Multivariable analysis in ENZAMET again shows assoc. between HSD3B1 and OS remains even after accounting for other clinical variables.
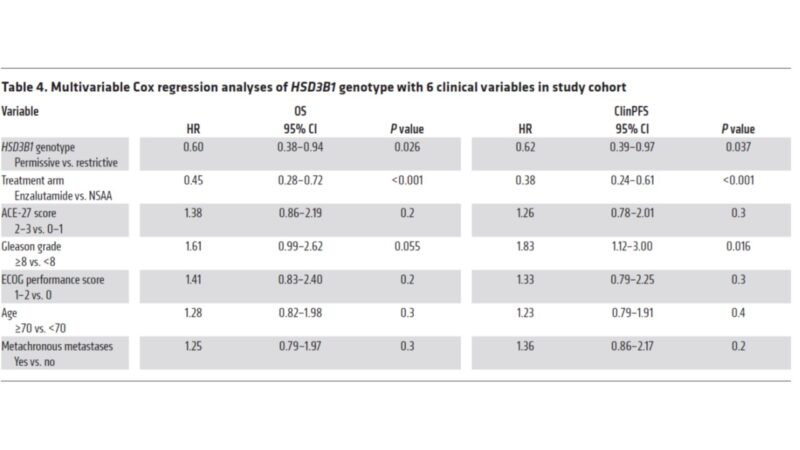
To be clear, analysis included only men treated with ADT+AR blocker, not dct. Dct treated men were excluded, thus bias possible. We did a related HSD3B1 analysis in ARCHES (no taxanes) but LV mHSPC follow up was not mature enough to draw conclusions. (link)
Take away? These are additional level 1 data that HSD3B1 is a clinically meaningful biomarker of hormonal therapy outcomes in LV mHSPC. With ADT only, HSD3B1 genetically enabling adrenal androgen use lowOS [E3805]; blocking effects of adrenal androgens upfront high OS [ENZAMET].
Germline HSD3B1 is easy to assess and common. We know the mechanism. Provides independent information. It is an invisible driver of pca mortality. Earlier use of hormonal therapy begs assessment in trials of earlier prostate cancer disease states. (link)
This also teaches us about physiology of pca. HSD3B1 allows pca to use adrenal steroids for CRPC. ENZAMET suggests HSD3B1 also confers adrenal androgen-dependence – likely earlier than thought possible. Can we apply lessons to localized pca?
Finally, I’m incredibly grateful to Ian Davis, Christopher Sweeney, Hui-Ming Lin, Lisa Horvath, the rest of the ANZUP group, Robert Diaz, Evan Roberts, all lab members, Desai Sethi Urology Institute and Sylvester Comprehensive Cancer Center! Also to PCF Science, NCI and CDMRP for support!”
Source: Nima Sharifi/X
More posts featuring Nima Sharifi on oncodaily.com
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
