The advent of immune checkpoint inhibitors (ICIs) has transformed the prognosis of advanced melanoma, markedly improving progression-free and melanoma-specific survival (MSS), especially in patients with high tumor mutational burden (TMB) or BRAF-mutated disease. In the adjuvant setting, ICIs significantly prolong relapse-free survival (RFS) and distant metastasis-free survival (DMFS); however, robust evidence linking adjuvant ICI therapy to improved MSS remains limited.
Previous studies have suggested that TMB and BRAF mutation status may be predictive of response to ICIs in advanced disease, and more recently, in the adjuvant setting for RFS. This study aimed to extend prior findings by examining the effect of TMB and BRAF mutation status on MSS in a well-defined cohort of patients with stage IIIC–IV melanoma receiving adjuvant anti–PD-1 therapy.
Authors: Julia Forschner, Julia Huynh, Christopher Schroeder, Sorin Armeanu-Ebinger, Olga Seibel-Kelemen, Axel Gschwind, Irina Bonzheim, Thomas K. Eigentler, Teresa Amaral, Stephan Ossowski, Lukas Flatz, Claus Garbe, Andrea Forschner, Markus Reitmajer
TMB and BRAF Status as Prognostic Markers in Stage IIIC–IV Melanoma on Adjuvant Anti–PD-1
This retrospective single-center study included all patients with stage IIIC, IIID, or IV melanoma who initiated adjuvant anti–PD-1 therapy (nivolumab or pembrolizumab) at the University Hospital Tübingen between March 2018 and September 2019, and for whom tumor tissue was available for next-generation sequencing (NGS). Patients with stage IIIA or IIIB disease were excluded due to insufficient tumor material for NGS. Treatment decisions followed the German melanoma guidelines and were made by an interdisciplinary tumor board.
Tumor and matched blood samples underwent NGS using a 700-gene panel. TMB was calculated as the number of nonsynonymous somatic variants per megabase; “TMB-high” was defined as ≥20 variants/megabase (top 20% of the cohort). BRAF V600E/K mutation status was determined as part of the same sequencing workflow.
RFS was defined as the time from first ICI dose to melanoma recurrence, melanoma-related death, or last follow-up. MSS was defined as the time from first ICI dose to melanoma-related death or last follow-up. Associations between clinicopathologic variables, TMB, BRAF status, and survival outcomes were evaluated by univariate and multivariate Cox regression analyses. Survival curves were generated using Kaplan–Meier methodology, and comparisons between groups were made using the log-rank test.
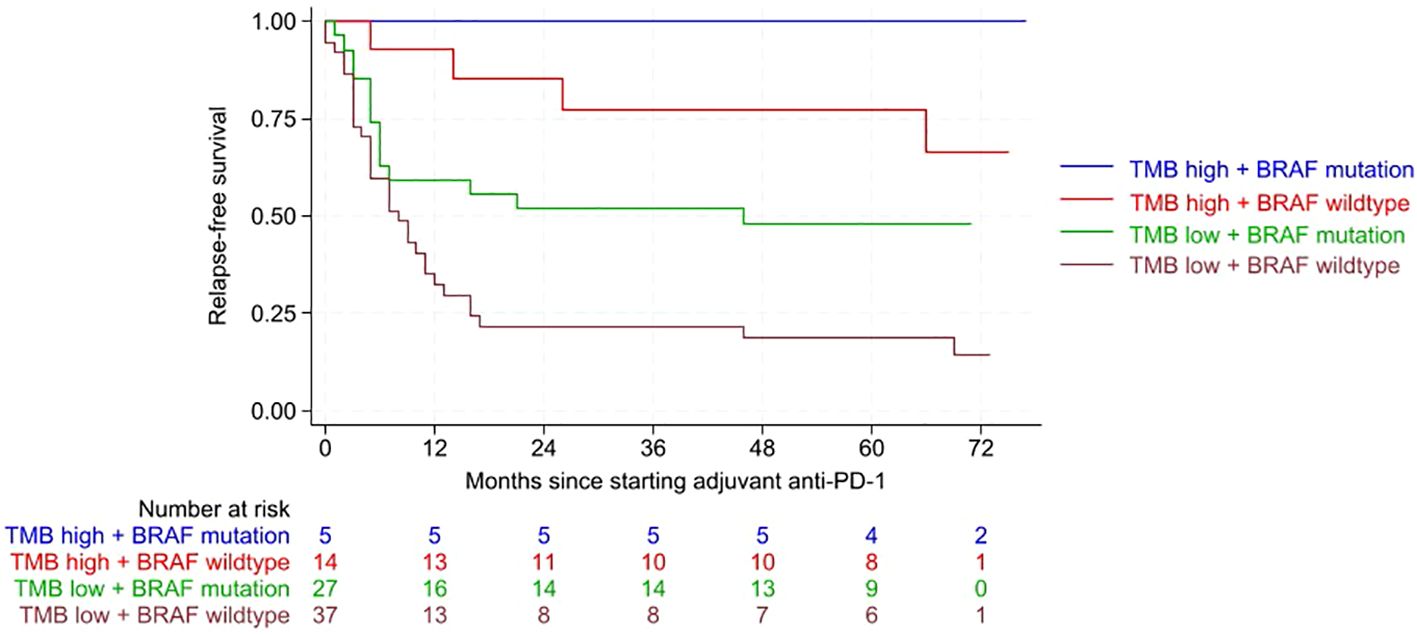
High TMB and BRAF Mutation Linked to Better Outcomes in Advanced Melanoma on Adjuvant Anti–PD-1
Of 165 patients who began adjuvant anti–PD-1 therapy during the study period, 83 met eligibility criteria. The median age was 65 years; 42% were female. Cutaneous melanoma was the most common subtype (75%), followed by occult (15%), acral (6%), and mucosal (5%) melanoma. Most patients (82%) had stage IIIC disease at treatment initiation; 6% had stage IIID and 12% had stage IV with no evidence of disease. Median follow-up was 59 months.
During follow-up, 59% of patients experienced relapse—31% locoregional and 28% distant. The relapse rate was higher than in the KEYNOTE-054 and CheckMate 238 trials, reflecting the more advanced disease stage of the cohort.
Patients with high TMB and BRAF-mutated tumors had the most favorable RFS (p<0.001). In multivariate analysis, BRAF wildtype tumors were associated with over twice the risk of relapse compared with BRAF-mutated tumors (HR 2.28, p=0.010). Low TMB conferred a sixfold higher risk of relapse versus high TMB (HR 6.24, p<0.001). Both TMB and BRAF status were independent predictors of RFS.
High TMB and BRAF mutation were also strongly associated with improved MSS (p=0.002). In multivariate analysis, low TMB conferred an approximately sixfold increased risk of melanoma-specific death (HR 5.70, p=0.018), while BRAF wildtype status doubled the risk (HR 2.34, p=0.051).
Other patient factors—sex, tumor stage at treatment initiation, and immune-related adverse events—did not significantly influence MSS.
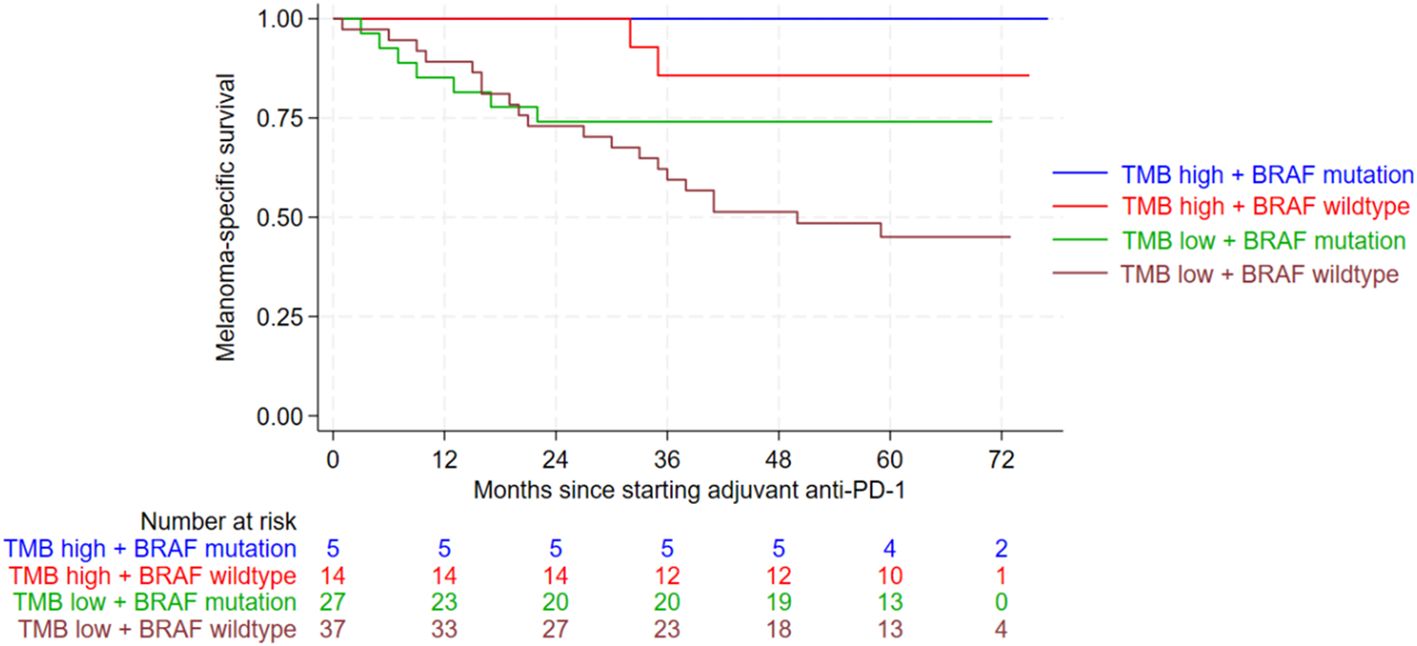
Discussion
This study provides novel evidence that high TMB combined with BRAF mutation predicts superior MSS in stage IIIC–IV melanoma patients treated with adjuvant ICIs. These findings parallel previous observations in the metastatic setting, reinforcing TMB as a strong, independent predictive and potentially prognostic biomarker in melanoma.
The biological rationale for this association is compelling: high TMB increases the neoantigen load, potentially enhancing ICI-mediated immune recognition, while BRAF mutations may interact with tumor biology and the immune microenvironment in ways that potentiate checkpoint blockade efficacy.
The higher relapse rate in this cohort compared to pivotal adjuvant ICI trials is likely attributable to the exclusive inclusion of higher-stage patients (IIIC–IV). The proportion of distant relapses was consistent with prior studies, but locoregional relapses were notably more frequent, suggesting that intensified local control strategies may be warranted in this subgroup.
While BRAF mutation status remains an established predictive marker for targeted therapy, its prognostic role in the adjuvant immunotherapy context merits further exploration. Additionally, TMB-high/BRAF-mutated patients might derive differential benefit from adjuvant BRAF/MEK inhibition versus ICI, an important question for future prospective trials.
Clinical Implications and Future Directions
-
Patient Selection: TMB and BRAF status may be useful for stratifying stage IIIC–IV melanoma patients when considering adjuvant ICI.
-
Therapeutic Strategy: In TMB-low/BRAF-wildtype patients, closer surveillance, consideration of adjuvant radiotherapy, or alternative systemic strategies may be warranted.
-
Research Needs: Prospective validation is necessary to determine whether TMB-high/BRAF-mutated patients benefit more from ICI or targeted therapy, and to integrate these markers into multifactorial predictive models.
You can read the full abstract here


