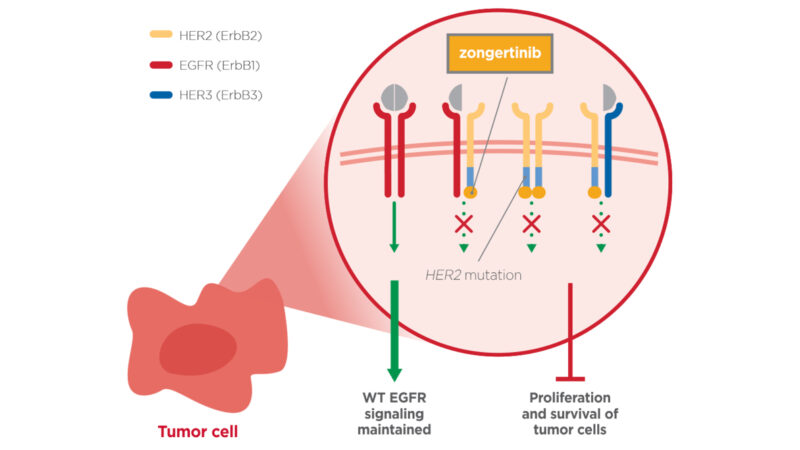
The FDA has recently granted Zongertinib, a HER2-specific tyrosine kinase inhibitor (TKI), Breakthrough Therapy Designation (BTD) for treating adult patients with advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC) with HER2 mutations who have previously undergone systemic therapy.
Estela Rodriguez shared a post on X:
“FDA Grants Zongertinib Breakthrough Therapy Designation in HER2-Mutant NSCLC.
Eagerly awaiting for more options for patients with HER2+ NSCLC post T-DXd.
BAY 2927088 also has FDA breakthrough designation.”
Estela (Estelamari) Rodriguez is Associate Director of Community Outreach and Co-Lead of Thoracic Site Disease Group of the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine. She is board certified in medical oncology and hematology. She has a special interest in the early detection of lung cancer and increasing access to clinical trials.
