Elisa Agostinetto shared a post on X about a recent paper by Benedikt Westphalen et al. published in Annals of Oncology.
Authors: Benedikt Westphalen, Diogo Martins Branco, George Pentheroudakis, Vivek Subbiah et al.
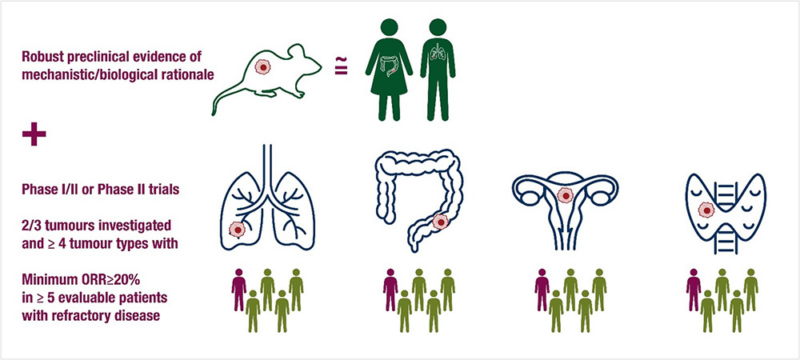
“Out in Annals of Oncology the position paper by ESMO on the landscape of tumour-agnostic authorised indications by FDA and EMA.
Tumor agnostic therapies must meet a minimum set of requirements to be eligible for tumour-agnostic potential.”
Source: Elisa Agostinetto/X
More posts by Elisa Agostinetto on oncodaily.com
Dr. Elisa Agostinetto, is a medical oncologist, specializing in breast cancer research. Elisa Agostinetto is a Medical Research Fellow at the University Hospital of Brussels since November 2021. Additionally, she has serves as a Clinical Research Fellow at Jules Bordet Institute. Her work focuses on clinical research in oncology, likely involving various aspects of cancer treatment and patient care.
Actively engaged in professional societies like ESMO, ASCO, and BSMO, she also contributes as a young investigator in the EORTC Breast Cancer Working Group and as a Committee member of the Young Cancer Professionals within the European Cancer Organization.
