In the rapidly evolving landscape of oncology, we are witnessing remarkable advancements in various treatment modalities that promise to reshape cancer care. Our editorial team has identified ten future cancer drugs that, while not yet FDA-approved, have garnered significant attention due to their impressive preliminary data and potential to offer innovative solutions for a range of malignancies.
These promising candidates, each with the potential to transform patient care through novel treatment approaches, signal a possible paradigm shift in how we address this complex group of diseases.
This article explores botensilimab, one of the drugs featured in OncoDaily’s “10 Most Promising Cancer Drugs Not Yet Approved: Solid Tumors – 2024 edition,” showcasing its potential to revolutionize treatment for a wide range of solid tumors, particularly those traditionally resistant to immunotherapy.
About Botensilimab
Botensilimab (BOT) is an investigational Fc-enhanced, multifunctional anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) antibody being developed by Agenus Inc. for the treatment of various solid tumors. As a next-generation CTLA-4 inhibitor, botensilimab represents a promising new approach to enhancing anti-tumor immune responses, particularly in “cold” tumors that have historically been resistant to immunotherapy.
This article provides an in-depth look at the mechanism of action, preclinical data, clinical trial results, and ongoing development of botensilimab as a potential best-in-class CTLA-4 inhibitor.
About Agenus Inc.
Agenus Inc. is a clinical-stage immuno-oncology company focused on the discovery and development of therapies that engage the body’s immune system to fight cancer. Founded in 1994 and headquartered in Lexington, Massachusetts, Agenus has built a diverse pipeline of immune-modulating antibodies, cancer vaccines, and adoptive cell therapies.
The company’s approach to cancer immunotherapy is multifaceted, encompassing checkpoint inhibitors, bispecific antibodies, cell therapies, cancer vaccines, and adjuvants. Botensilimab, the company’s lead candidate, exemplifies its commitment to developing innovative immunotherapies to address unmet medical needs in oncology.
Botensilimab Mechanism of Action
CTLA-4 is a key immune checkpoint molecule that acts as a negative regulator of T cell activation. It competes with CD28 for binding to the costimulatory molecules CD80 and CD86 on antigen-presenting cells, thereby inhibiting T cell proliferation and function.
CTLA-4 blockade has emerged as a powerful strategy to enhance anti-tumor immunity, with the first-generation anti-CTLA-4 antibody ipilimumab demonstrating significant clinical benefit in melanoma and other cancers.
Botensilimab represents a next-generation approach to CTLA-4 inhibition, with several key features that distinguish it from first-generation agents:
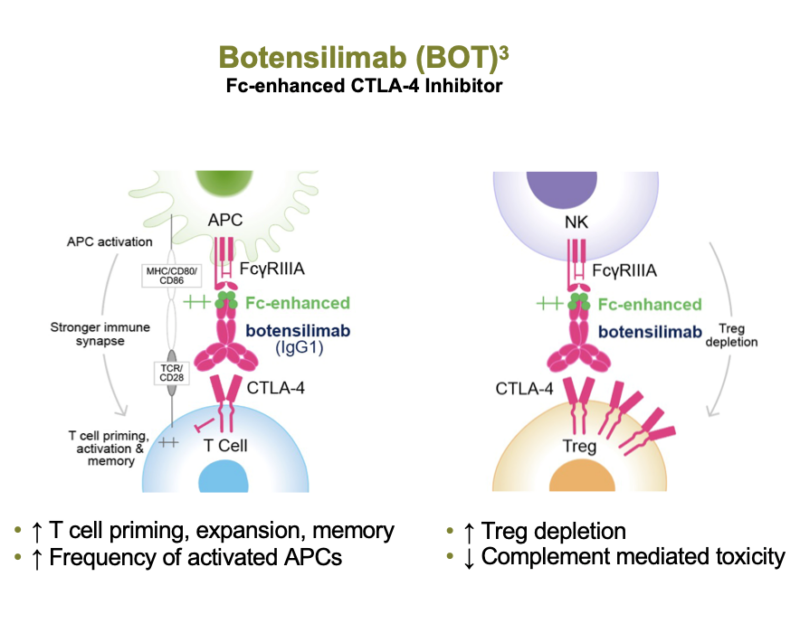
- Fc Enhancement: Botensilimab’s Fc region has been engineered to improve binding to activating Fcγ receptors on effector cells, particularly natural killer (NK) cells and macrophages. This enhancement leads to:
- More efficient antibody-dependent cellular cytotoxicity (ADCC) against regulatory T cells (Tregs) in the tumor microenvironment
- Enhanced T cell priming and expansion through improved interactions with antigen-presenting cells
- Multifunctionality: Beyond CTLA-4 blockade, botensilimab exhibits additional immunomodulatory effects:
- Increased activation of dendritic cells and other antigen-presenting cells
- Enhanced production of pro-inflammatory cytokines
- Modulation of the tumor microenvironment to promote a more immunogenic state
- Improved Binding Across FcγRIIIA Variants: Approximately 40% of the population has low-affinity FcγRIIIA receptors, which can limit the efficacy of first-generation CTLA-4 inhibitors. Botensilimab is designed to bind effectively to all variants of FcγRIIIA, potentially expanding the population of responders.
- Reduced Complement-Mediated Toxicity: The Fc modification of botensilimab is engineered to avoid binding to complement proteins, potentially reducing inflammatory side effects associated with complement activation.
Key features of botensilimab’s mechanism include:
- High affinity binding to CTLA-4, effectively blocking its interaction with CD80/CD86
- Enhanced Treg depletion in the tumor microenvironment through improved ADCC
- Potent stimulation of effector T cell proliferation and activation
- Modulation of the tumor microenvironment to promote a more immunogenic state
- Potential for improved safety profile due to reduced complement activation
Preclinical Data
In preclinical studies, botensilimab demonstrated potent anti-tumor activity across a range of solid tumor models. Key findings from these studies include:
- Superior Treg depletion in tumor tissue compared to first-generation CTLA-4 inhibitors
- Enhanced T cell infiltration and activation in traditionally “cold” tumor models
- Synergistic activity when combined with PD-1 blockade or other immunomodulatory agents
- Favorable pharmacokinetic properties, including extended half-life and high tumor penetration
- Improved safety profile in non-human primate studies, with reduced incidence of immune-related adverse events
Notably, botensilimab showed activity in preclinical models of tumors that have historically been resistant to checkpoint inhibition, including microsatellite stable (MSS) colorectal cancer and pancreatic cancer.
Clinical Development and Results
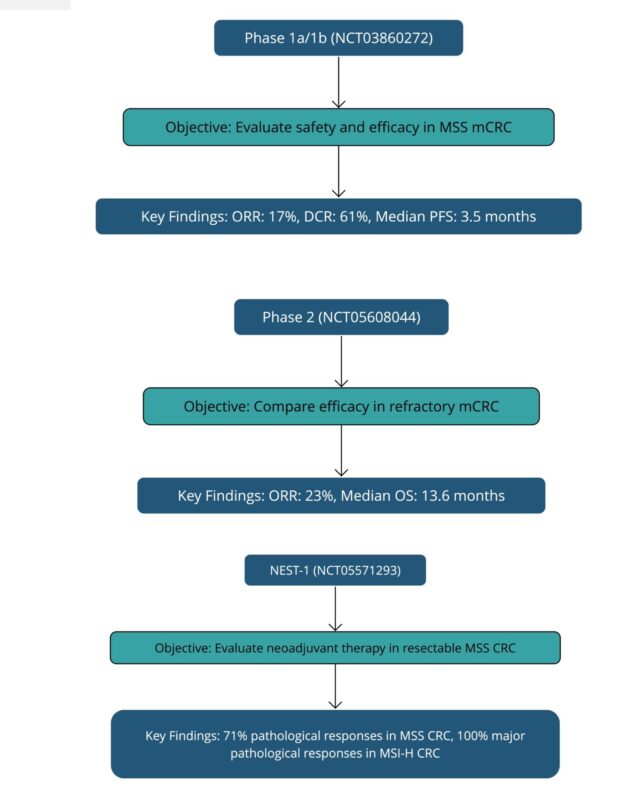
Phase 1/2 Studies
The first-in-human study of botensilimab (NCT03860272) was a phase 1/2 dose-escalation and expansion trial in patients with advanced solid tumors. The study enrolled patients across multiple cohorts, including microsatellite stable metastatic colorectal cancer (MSS mCRC), a tumor type known to be resistant to current immunotherapies.
Key findings from this study included:
- Safety and Tolerability:
- Treatment-related adverse events (TRAEs) occurred in 89% of patients (131/148)
- Most common TRAEs: fatigue (35%), diarrhea (32%), and pyrexia (24%)
- Grade ≥3 TRAEs occurred in 26% of patients
- No treatment-related deaths reported
- Treatment discontinuation due to TRAEs: 12% (18/148)
- Efficacy in MSS mCRC (n=101 evaluable patients):
- Objective Response Rate (ORR): 17% (17/101; 95% CI, 10–26%)
- Disease Control Rate (DCR): 61% (62/101; 95% CI, 51–71%)
- Median Duration of Response (DOR): Not reached (95% CI, 5.7 months–NR)
- Median Progression-Free Survival (PFS): 3.5 months (95% CI, 2.7–4.1 months)
- Median Overall Survival (OS): 21.2 months (95% CI, 16.5–NR)
- Efficacy across different metastatic sites in MSS mCRC:
- Lung involvement: 26% ORR (16/62), median OS 21.2 months (20.7–NR)
- Peritoneal involvement: 18% ORR (6/33), median OS 20.7 months (6.3–NR)
- Soft tissue involvement: 27% ORR (4/15), median OS 20.7 months (3.6–NR)
- Other organ involvement: 33% ORR (6/18), median OS 20.9 months (6.3–NR)
These results are particularly noteworthy given the historical resistance of MSS mCRC to immunotherapy, with previous studies of checkpoint inhibitors showing ORRs of less than 5% in this population.
Neoadjuvant Studies
The potential of botensilimab is also being explored in the neoadjuvant setting through the NEST-1 trial (NCT05571293) in resectable colorectal cancer. Preliminary results have shown:
- MSS colon cancer (n=17):
- Pathological response rate (>50% regression): 71% (12/17)
- Complete pathological response rate: 35% (6/17)
- MSI-H colon cancer (n=3):
- Major pathological response rate: 100% (3/3)
- No surgery delays due to treatment-related adverse events
These encouraging data suggest that botensilimab may have a role in the neoadjuvant setting, potentially reducing the need for extensive surgery or adjuvant chemotherapy in select patients.
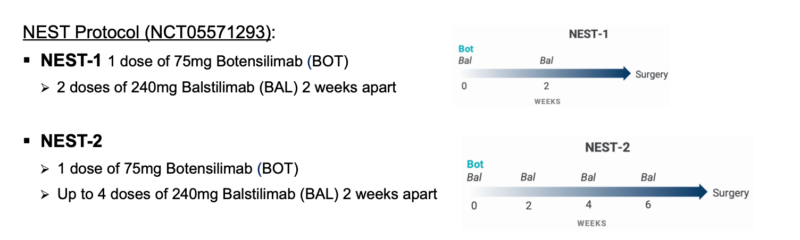
The NEST-2 cohort of the trial (NCT05571293) further explored the potential of botensilimab in the neoadjuvant setting with an extended treatment regimen. Preliminary results for this cohort showed:
- MSS colon cancer (n=9):
- Pathological response rate (>50% regression): 78% (7/9)
- Complete pathological response rate: 56% (5/9)
- MSI-H colon cancer (n=1)
- Complete pathological response rate: 100% (1/1)
Increased response rates compared to NEST-1, potentially due to more doses of balstilimab and a longer interval to surgery.
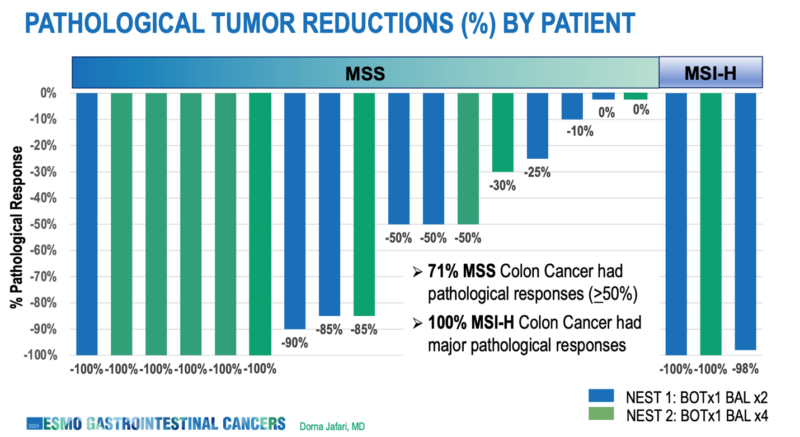
These results from NEST-2 suggest that an extended neoadjuvant regimen of botensilimab plus balstilimab may lead to even higher response rates, particularly in MSS colon cancer. The impressive complete pathological response rate of 56% in MSS tumors is especially noteworthy, as these cancers have traditionally been less responsive to immunotherapy approaches.
Ongoing Clinical Trials
Several additional clinical trials of botensilimab are currently ongoing or planned:
- Phase 2 randomized trial: Botensilimab ± Balstilimab (anti-PD-1) vs. standard of care in MSS mCRC (NCT05608044, fully enrolled)
- Phase 2 trial: Botensilimab + Balstilimab (anti-PD-1) in 1st line NSCLC (NCT06322108, fully enrolled)
- Planned global phase 3 trial in MSS mCRC
- Expansion into other indications:
- Melanoma
- Cervical cancer
- Pancreatic cancer
- Non-small cell lung cancer
- Combination strategies:
- With chemotherapy
- With targeted therapies
- With other immunomodulatory agents
Unique Features and Potential Advantages
Several characteristics of botensilimab suggest it may have advantages over first-generation CTLA-4 inhibitors:
- Enhanced Treg Depletion: The Fc-enhancement of botensilimab leads to more efficient depletion of immunosuppressive Tregs in the tumor microenvironment.
- Improved T Cell Priming: Botensilimab’s multifunctional nature may result in more robust T cell activation and proliferation.
- Activity in “Cold” Tumors: Preliminary clinical data suggest efficacy in traditionally immunotherapy-resistant tumors like MSS mCRC.
- Potential for Improved Safety: The engineered Fc region may reduce complement-mediated toxicities associated with first-generation CTLA-4 inhibitors.
- Broader Population Benefit: Improved binding across FcγRIIIA variants may extend efficacy to a larger proportion of patients.
- Synergistic Potential: Preclinical and early clinical data support combination strategies with other immunotherapies or targeted agents.
Development Status and Future Directions
The FDA has granted Fast Track Designation to the combination of botensilimab (AGEN1181) and balstilimab (AGEN2034) for treating non-MSI-H and dMMR metastatic colorectal cancer without active liver involvement. This designation expedites the review process and highlights the therapy’s potential to address unmet medical needs.
While not yet fully approved, botensilimab is progressing through late-stage clinical trials, including a global phase 2 study (NCT05608044) and a planned phase 3 trial. The Fast Track status may lead to Priority Review, potentially accelerating the drug’s path to market.
As Agenus Inc. advances its development program, patients and healthcare providers anticipate further progress in the FDA approval process for this promising immunotherapy combination.
Future development plans for botensilimab include:
- Completion of ongoing phase 2 and initiation of phase 3 trials in MSS mCRC
- Expansion into additional tumor types and earlier lines of therapy
- Evaluation of novel combination strategies to enhance efficacy and overcome resistance
- Exploration of biomarkers to identify patients most likely to benefit from treatment
- Investigation of botensilimab in the neoadjuvant and adjuvant settings
Conclusion
Botensilimab represents a significant advancement in the field of cancer immunotherapy. Its innovative, multifunctional mechanism of action, designed to overcome the limitations of first-generation CTLA-4 inhibitors, has shown remarkable promise in traditionally immunotherapy-resistant tumors like MSS mCRC. The encouraging efficacy data, coupled with a manageable safety profile, position botensilimab as a potential game-changer in oncology.
As clinical trials progress and more data become available, the oncology community eagerly awaits further validation of botensilimab’s potential. If approved, this novel agent could expand the reach of immunotherapy to a broader population of cancer patients, offering new hope for those with limited treatment options.
The ongoing research into botensilimab exemplifies the rapid pace of innovation in cancer therapeutics and the continued pursuit of more effective, personalized treatment strategies.
As we look to the future, botensilimab stands as a testament to the power of scientific innovation and the potential for transformative breakthroughs in the fight against cancer.
While challenges remain, the promise of this novel therapy offers renewed hope for patients and oncologists alike, paving the way for a new era in cancer immunotherapy.


