At ESMO 2025, Huaqiang Zhou (Guangzhou, China) presented the Iza-Bren data during the Proffered Paper session on Developmental Therapeutics. He shared key insights from the preplanned interim analysis of BL-B01D1-303, a randomized phase III study testing the EGFR×HER3 bispecific antibody-drug conjugate against treatment/physician’s choice chemotherapy in heavily pretreated recurrent/metastatic nasopharyngeal carcinoma (R/M-NPC). The headline findings were higher objective response rate (ORR), longer progression-free survival (PFS), and more durable responses with Iza-Bren versus chemotherapy, alongside a manageable, mainly hematologic safety profile.
Introduction
Patients with R/M-NPC who progress after multiple lines of chemotherapy and PD-(L)1 inhibitors face limited options and poor prognosis. Iza-Bren, an EGFR×HER3 bispecific ADC, is designed to co-target two relevant receptors in NPC biology and deliver a cytotoxic payload to tumor cells. BL-B01D1-303 evaluates whether this dual-targeting ADC can outperform standard chemotherapy choices in late-line R/M-NPC.
Methods
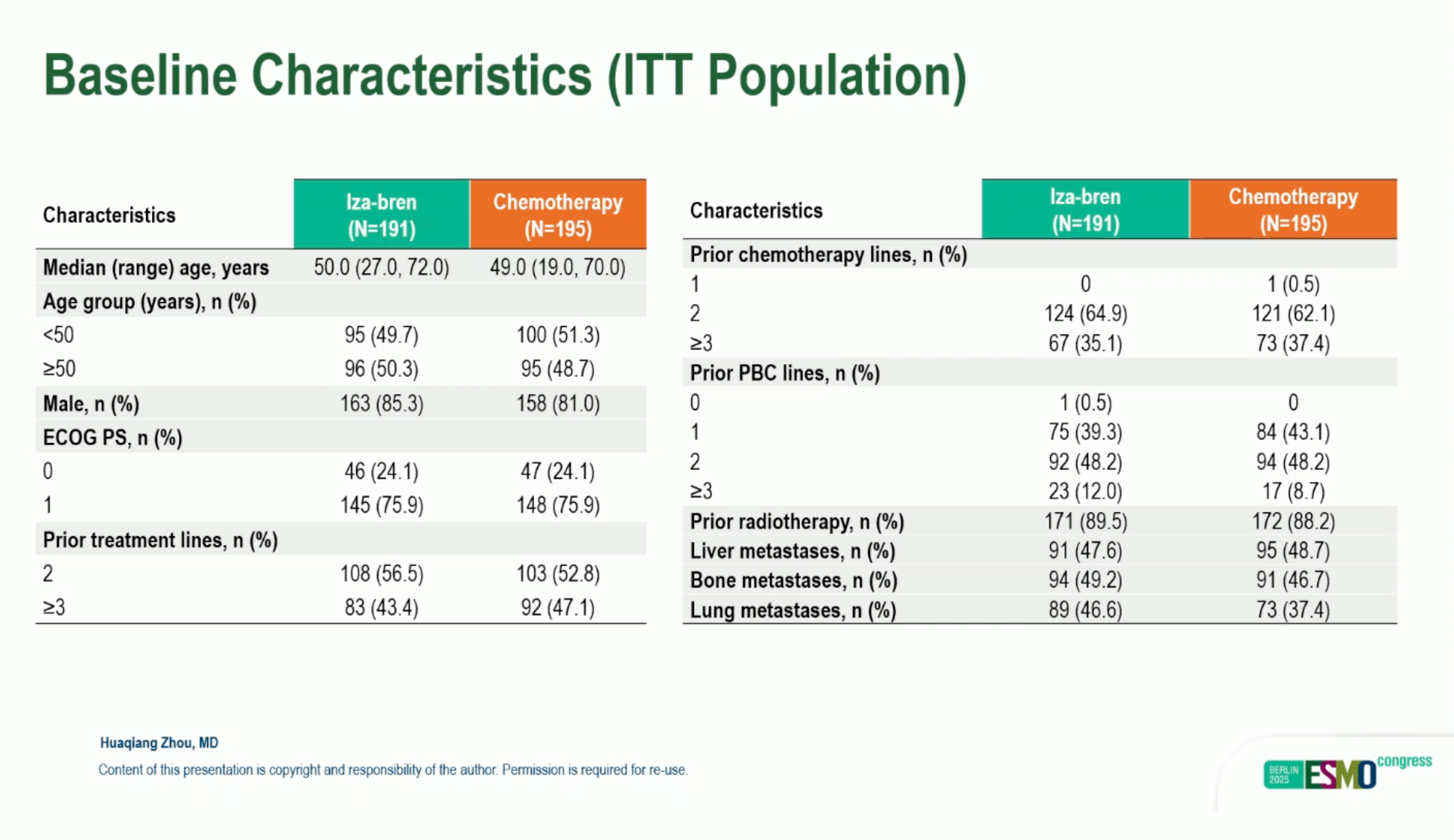
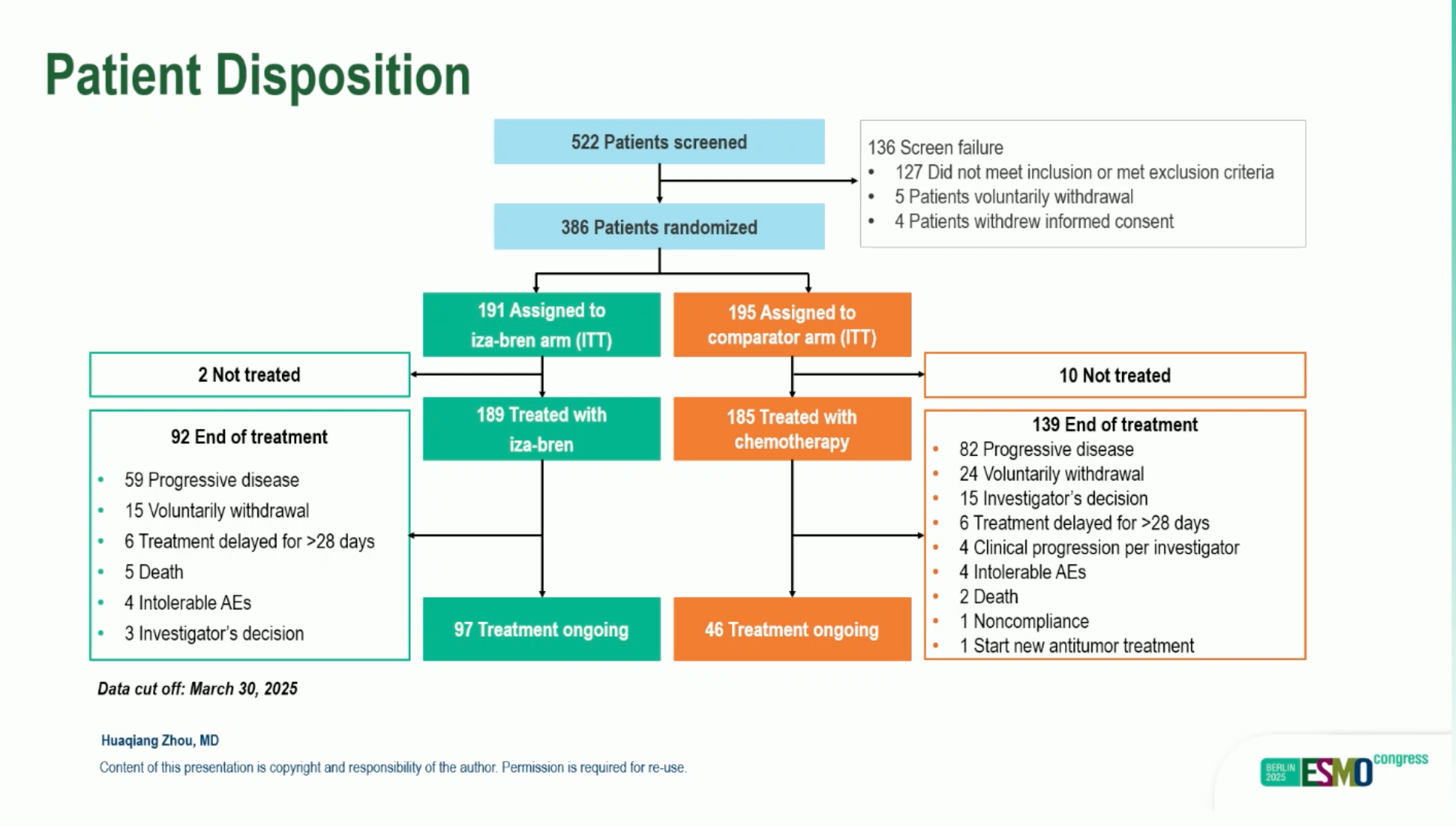
This open-label, multicenter, randomized (1:1) phase III trial enrolled adults with R/M-NPC after ≥2 prior chemotherapy lines (including ≥1 platinum-based) and prior PD-(L)1 therapy. The preplanned interim analysis (IA)used a data cutoff of March 30, 2025. Between December 4, 2023 and February 21, 2025, 386 patients were randomized (Iza-Bren n=191; chemotherapy n=195). Dual primary endpoints were confirmed ORR (cORR; RECIST 1.1, blinded independent central review [BICR]) and overall survival (OS); duration of response (DoR), PFS, and safety were secondary endpoints. Median follow-up at IA was 7.66 vs 7.10 months for Iza-Bren and chemotherapy, respectively. ClinicalTrials.gov: NCT06118333.
Arms & dosing
- Iza-Bren: 2.5 mg/kg on day 1 and day 8 every 3 weeks
- Treatment/physician’s choice (TPC): capecitabine, gemcitabine, or docetaxel
Results
From Dec 4, 2023 to Feb 21, 2025, 386 patients were randomized (Iza-Bren n=191; TPC n=195). At the interim analysis (data cutoff Mar 30, 2025), median follow-up was 7.66 vs 7.10 months (Iza-Bren/TPC).
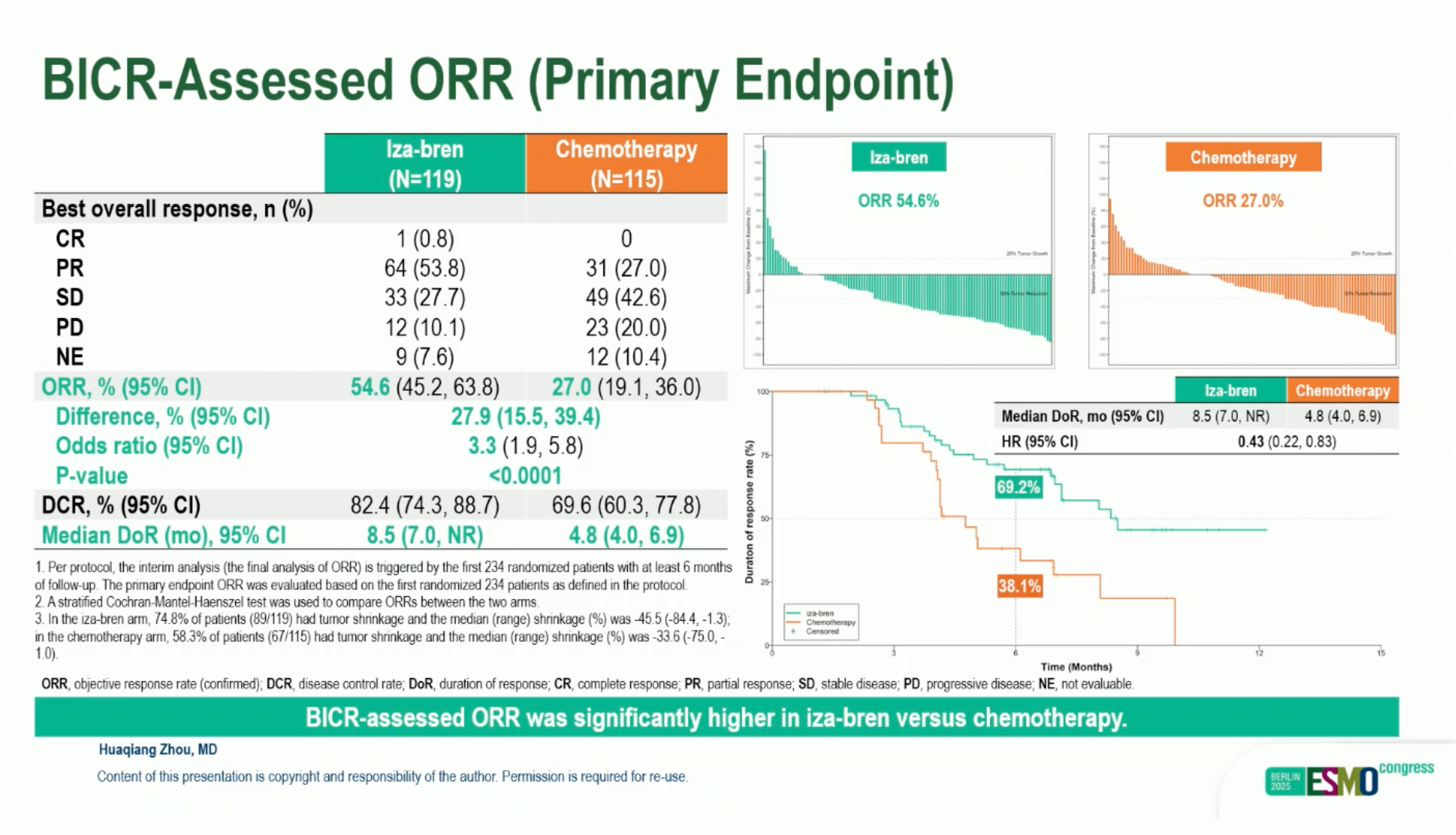
- cORR: 54.6% vs 27.0% (Iza-Bren vs TPC); odds ratio 3.33; P < .0001.
- Median DoR: 8.51 vs 4.76 months.
- Median PFS: 8.38 vs 4.34 months; HR 0.44 (95% CI 0.32–0.62).
- OS: Immature at IA.
- Grade ≥3 TRAEs: 79.9% (Iza-Bren) vs 61.6% (TPC).
Predominantly hematologic with Iza-Bren; non-hematologic events largely grade 1–2.
Take-home messages
- Iza-Bren more than doubled ORR and nearly doubled median PFS vs chemotherapy, with longer DoR.
- 56% lower risk of progression or death (HR 0.44).
- Higher grade ≥3 hematologic toxicity but manageable overall; requires proactive monitoring/supportive care.
- OS not mature—continued follow-up will define survival benefit and durability.
- If benefits persist with mature OS and QoL data, Iza-Bren is a strong candidate for a new standard in heavily pretreated R/M-NPC (NCT06118333).
The ESMO presentation was accompanied by a simultaneous online publication in The Lancet.
You can also read the Iza-Bren FDA Breakthrough Therapy Designation article on OncoDaily.

Follow ESMO 2025 live by checking the official program, abstracts, and announcements on the ESMO website, and get rolling highlights, summaries, expert takes, and Special Live Coverage on OncoDaily.