Armelle Dufresne from Centre Léon Bérard presented the COTESARC Trial data at ESMO 2025 in Berlin. She outlined the multicentre, open-label phase I–II evaluation of cobimetinib (MEK inhibitor) + atezolizumab (PD-L1 inhibitor) across soft-tissue sarcoma (STS) cohorts and summarized efficacy, safety, and reasons for early termination. 
Introduction
Immune checkpoint inhibitors (ICIs) as monotherapy have yielded modest activity in unselected STS, likely reflecting low tumor immunogenicity and an immune-excluded microenvironment. Preclinical data suggest that MEK inhibition can increase tumor antigen presentation, T-cell infiltration, and PD-L1 expression, potentially sensitizing STS to PD-(L)1 blockade. COTESARC tested this biology-driven strategy in the clinic: pairing cobimetinib with atezolizumab across genomically defined STS cohorts. The trial used a Bayesian, cohort-wise futility rule based on the progression-free rate at 16 weeks (PFR-16W) to decide whether signals justified continued enrollment. Secondary endpoints covered safety, overall response (ORR), progression-free survival (PFS), and overall survival (OS), alongside exploratory biomarker profiling (MSI, MAPK activation, tumor mutational burden [TMB]) via Foundation Medicine.
Study design
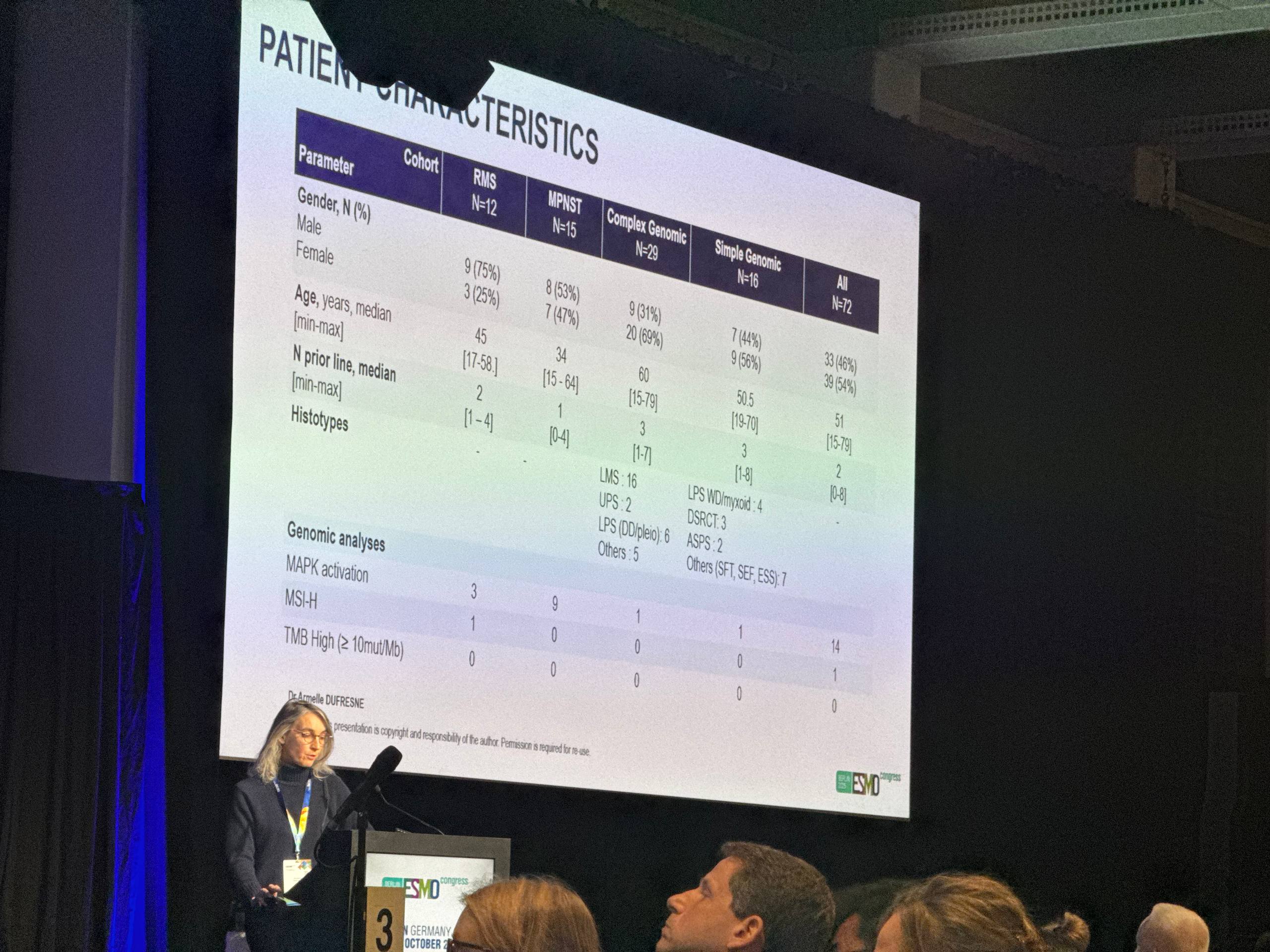
COTESARC was a multicentre, open-label, phase I–II study evaluating cobimetinib plus atezolizumab in four prespecified STS cohorts: complex genomic (CG) STS, single genomic (SG) STS, malignant peripheral nerve sheath tumor (MPNST), and rhabdomyosarcoma (RMS). Molecular annotations included MSI-H status, MAPK pathway activation, and TMB. The primary decision metric was PFR-16W, analyzed sequentially in blocks of 10 patients per cohort using a Bayesian approach; cohorts stopped for futility when the probability that PFR-16W ≤30% was high. Key secondary endpoints were safety, ORR, PFS, and OS.
Cobimetinib 60 mg PO QD days 1–21 q28d + Atezolizumab 840 mg IV q2w
Primary endpoint is PFR-16W (Bayesian sequential monitoring with futility if Pr[PFR-16W ≤30%] high), and secondary endpoints are safety, ORR, PFS, and OS.
Results
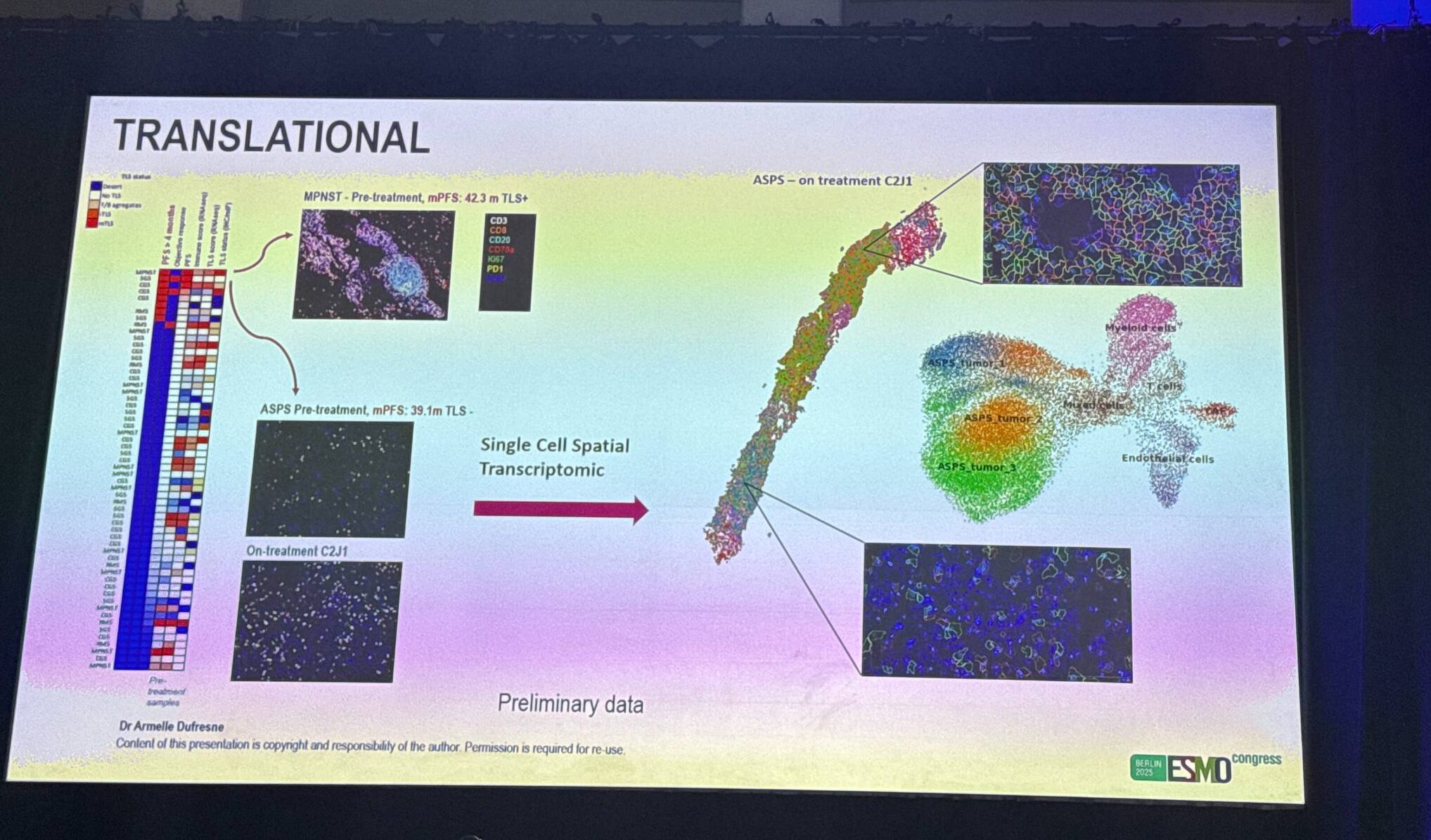
The trial was stopped early for futility and an unfavourable benefit–to–risk signal driven by myocarditis (5 grade 2–3 cases). Across cohorts, PFR-16W did not meet the 30% benchmark (high futility probabilities), median PFS was ~1.8 months, and median OS was limited (≈3.6–8.2 months). A small subset showed durable benefit: ASPS (PFS 39.1 m, R0 of metastases), MAPK+ MPNST (42.3 m) and RMS (16.3 m), and two CG cases (UPS 33.7 m; undifferentiated 18.7 m). No TMB-high tumors; one MSI-H RMS had no benefit. Overall, the combination did not demonstrate population-level synergy in unselected STS, though biology-defined sensitivity (e.g., MAPK activation) may explain the rare outliers.
Key take-home messages
- Cobimetinib + atezolizumab failed to clear the PFR-16W bar in all cohorts and showed short median PFS, supporting no overall synergy in unselected STS.
- Grade 2–3 myocarditis occurred in five patients; careful cardiac monitoring is essential if exploring this doublet further.
- Prolonged responders across histotypes—particularly with MAPK activation—suggest that biomarker-driven enrichment (and deeper immune/transcriptomic profiling) may identify rare sensitive subsets.
- Ongoing translational analyses (spatial transcriptomics, RNA-seq, multiplex immunofluorescence) aim to uncover mechanisms of response and resistance to guide smarter, safer future trials.
You Can Follow ESMO 2025 live by checking the official program, abstracts, and announcements on the ESMO website, and get rolling highlights, summaries, expert takes, and Special Live Coverage on OncoDaily


