“My doctors estimated that I had an 87 percent risk of breast cancer and a 50 percent risk of ovarian cancer… Once I knew that this was my reality, I decided to be proactive and to minimize the risk as much”
In 2013, Angelina Jolie made a personal medical decision public one that reshaped global conversations about genetic risk, cancer prevention, and patient autonomy. Her disclosure of carrying a BRCA1 gene mutation and undergoing a preventive bilateral mastectomy sparked what researchers and clinicians now call the “Angelina Jolie effect”: a measurable, sustained increase in genetic testing, cancer screening, and awareness worldwide.

Photo: Harald Krichel via Wikimedia Commons
More than a decade later, this moment is regarded as one of the clearest demonstrations of how responsible celebrity disclosure grounded in evidence and nuance can positively influence public health behavior.
The Disclosure That Changed Everything
On May 14, 2013, Jolie published an op-ed titled “My Medical Choice” in The New York Times. In it, she revealed a strong family history of breast and ovarian cancer and shared that genetic testing had identified a pathogenic BRCA1 mutation, placing her estimated lifetime risk of breast cancer at 87% and ovarian cancer at approximately 50%.
After consultation with specialists in oncology, genetics, and surgery, Jolie chose to undergo a risk-reducing bilateral mastectomy, lowering her estimated breast cancer risk to below 5%, consistent with outcomes reported in high-risk BRCA1 carriers.
In March 2015, she followed with a second op-ed, “Diary of a Surgery,” describing her decision to undergo preventive removal of her ovaries and fallopian tubes. Importantly, Jolie emphasized that her decision was personal, not prescriptive and that a positive genetic test does not automatically require surgery.
These disclosures demystified genetic testing and preventive oncology at a time when BRCA mutations were largely misunderstood outside medical circles.
What Are BRCA Mutations and Why Do They Matter?
BRCA1 and BRCA2 are tumor-suppressor genes that play a central role in maintaining genomic stability. They are essential components of homologous recombination, a high-fidelity DNA repair pathway responsible for correcting double-strand DNA breaks. When these genes function normally, they help prevent the accumulation of genetic errors during cell division. However, pathogenic variants in BRCA1 or BRCA2 disrupt this repair mechanism, allowing DNA damage to persist and increasing the likelihood of malignant transformation over time.
Photo: Depositphotos
As a result, individuals who carry germline BRCA mutations face a substantially elevated lifetime risk of developing certain cancers compared with the general population.
How Do BRCA Mutations Affect Cancer Risk?
Large prospective studies and meta-analyses have consistently shown that BRCA mutations are associated with markedly increased cancer risk, particularly for breast and ovarian malignancies. Women with BRCA1 mutations have an estimated lifetime breast cancer risk ranging from approximately 65% to 80%, while those with BRCA2 mutations face a risk of about 60% to 75%. Ovarian cancer risk is also significantly elevated, reaching roughly 35% to 45% among BRCA1 carriers and about 10% to 20% among BRCA2 carriers.
Beyond breast and ovarian cancer, BRCA mutations are linked to higher risks of other malignancies. These include male breast cancer, prostate cancer, pancreatic cancer, and melanoma, with the strongest associations observed in BRCA2 carriers. Despite these elevated risks, it is crucial to recognize that BRCA mutations account for only a minority of cancer cases overall. Approximately 5–10% of breast cancers and 10–15% of ovarian cancers are attributable to BRCA1 or BRCA2 mutations, meaning that most individuals diagnosed with these cancers do not carry a BRCA mutation (Kuchenbaecker et al., 2017; NCCN, 2024).
Does a BRCA Mutation Always Mean Cancer?
Although BRCA mutations are considered high-penetrance genetic variants, they are not deterministic. Penetrance varies widely between individuals and is influenced by multiple modifying factors, including reproductive history, hormonal exposures, lifestyle factors, polygenic background, and environmental influences. Consequently, not all BRCA mutation carriers will develop cancer during their lifetime, even without preventive intervention.
This distinction is critical in both clinical counseling and public communication, as it underscores that genetic risk represents probability rather than destiny.
How Should Cancer Risk Be Managed in Individuals With a BRCA Mutation?
Identifying a BRCA mutation has significant implications for personalized cancer prevention and treatment. For unaffected carriers, management often includes enhanced surveillance strategies, such as earlier initiation and increased frequency of breast MRI and mammography. In selected individuals, risk-reducing surgery including bilateral mastectomy and/or salpingo-oophorectomy—may be considered based on age, mutation type, family history, and personal preferences.
In certain contexts, chemoprevention may also be discussed. Importantly, BRCA status has therapeutic implications for patients who develop cancer. Tumors arising in the setting of BRCA deficiency are often sensitive to PARP inhibitors, which exploit synthetic lethality in homologous recombination–deficient cells and have become a cornerstone of targeted therapy in breast, ovarian, pancreatic, and prostate cancers.
Crucially, current guidelines emphasize that BRCA testing should be offered based on personal and family history or specific clinical criteria. It is not recommended as a population-wide screening tool, as indiscriminate testing may lead to unnecessary anxiety, misinterpretation of results, and inappropriate interventions.
The Angelina Jolie Effect: Awareness With Scientific Balance
Public understanding of BRCA mutations changed dramatically after Angelina Jolie publicly disclosed her BRCA1 mutation and her decision to undergo preventive surgery. Importantly, her messaging consistently reflected core principles supported by clinical evidence. She emphasized that most women do not carry BRCA mutations, that a positive test does not guarantee cancer development, and that preventive strategies must be individualized through genetic counseling and shared decision-making.
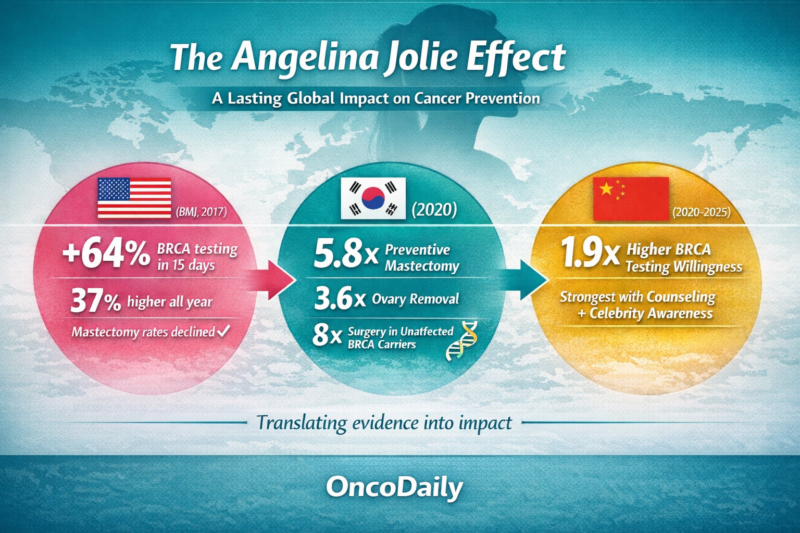
This balanced framing aligned closely with established clinical guidelines and helped prevent widespread panic or overreaction. Population-level studies conducted after her disclosure demonstrated increased referrals for genetic counseling and higher uptake of BRCA testing among appropriate risk groups, without a corresponding rise in unnecessary prophylactic surgery. These findings suggest that the “Angelina Jolie effect” represents a rare example of celebrity-driven awareness that achieved a net positive public health impact by encouraging informed, evidence-based engagement with cancer prevention (Evans et al., 2014; Desai & Jena, 2016).
You Can Also Read Miranda McKeon:Battling Breast Cancer at 19, Inspiring Millions on Instagram by OncoDaily

Written by Aharon Tsaturyan, MD, Editor at OncoDaily Intelligence Unit
FAQ
What is the “Angelina Jolie effect”?
The “Angelina Jolie effect” refers to the significant global increase in awareness, genetic counseling referrals, and BRCA testing that followed Angelina Jolie’s 2013 public disclosure of her BRCA1 mutation and preventive surgery. Multiple studies have shown sustained rises in appropriate genetic testing without increased unnecessary surgery.
Why did Angelina Jolie choose preventive mastectomy?
Angelina Jolie carried a pathogenic BRCA1 mutation, which gave her an estimated 87% lifetime risk of breast cancer and ~50% risk of ovarian cancer. After specialist consultation, she chose preventive surgery to dramatically reduce that risk, lowering her breast cancer risk to below 5%.
What are BRCA1 and BRCA2 mutations?
BRCA1 and BRCA2 are tumor-suppressor genes involved in repairing damaged DNA. Pathogenic mutations impair this repair process, increasing the risk of several cancers most notably breast and ovarian cancer.
Does having a BRCA mutation mean you will definitely get cancer?
No. A BRCA mutation increases cancer risk but does not guarantee cancer development. Many carriers never develop cancer, as risk is influenced by genetics, lifestyle, reproductive factors, and environmental exposures.
How much does a BRCA mutation increase cancer risk?
Women with BRCA1 mutations face a 65–80% lifetime risk of breast cancer and 35–45% ovarian cancer risk. For BRCA2, risks are approximately 60–75% for breast cancer and 10–20% for ovarian cancer. Risks for prostate, pancreatic cancer, and melanoma are also increased.
Should all women get BRCA genetic testing?
No. Current guidelines recommend BRCA testing for individuals with specific personal or family histories suggestive of hereditary cancer. Population wide screening is not recommended due to the risk of unnecessary anxiety and misinterpretation.
Did Angelina Jolie’s disclosure lead to unnecessary surgeries?
No. Studies show that while BRCA testing increased significantly, rates of preventive mastectomy did not rise disproportionately. In fact, data suggest testing expanded mainly among appropriately lower-risk women, reflecting informed decision-making rather than overtreatment.
How did the Angelina Jolie effect impact public health?
The effect improved awareness of hereditary cancer risk, normalized genetic counseling, and encouraged proactive but evidence-based prevention. It is considered one of the most successful examples of celebrity influence improving health behavior without causing harm.
How are BRCA mutations treated if cancer develops?
Cancers associated with BRCA mutations often respond well to PARP inhibitors, a targeted therapy that exploits defective DNA repair pathways. These drugs are now standard treatments in BRCA-related breast, ovarian, prostate, and pancreatic cancers.
What is the long-term legacy of the Angelina Jolie effect in oncology?
The legacy lies in patient empowerment, responsible risk communication, and precision prevention. Jolie’s disclosure demonstrated how personal storytelling when grounded in medical evidence can advance public health without distortion or panic.


