The WCLC24 is taking place at the San Diego Convention Center from September 7 to 10, 2024.
Organized by the International Association for the Study of Lung Cancer (IASLC), this major conference is gathering leading experts, researchers, and oncologists to present and discuss the latest advancements in lung cancer research, treatment, and personalized therapies, fostering collaborative efforts towards overcoming the challenges of lung cancer.
WCLC24 IASLC committee are rocking the social media and communications workshop !
Some highlights:
Estela Rodriguez : channel your inner Beyoncé
Mattew Smeltzer: demystify your data
Naomi Reguart: be the bridge between science and society.

Congrats to mentor, friend and colleague Julie Brahmer for her well-deserved IASLC Paul A. Bunn Scientific Award
Proud to be Team Julie.

Very special moment meeting my former patient Sydney Barned and her husband at IASLC WCLC24
We first met in 2017, when she was diagnosed with stage IV ALK+ NSCLC
She is now a practicing doctor patient advocate.

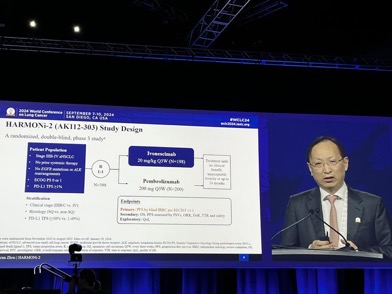
Ph III HARMONI-2 ivonescimab v pembro in PDL>1% NSCLC:
– 398pts
– PFS HR 0.51 (11.1 v 5.82 mPFS)
– benefit across histology and PDL1
Impressive data overall
Is this a step forward for PDL1 1-49% grp? 80% males enrolled, data needed in women
I think this is a key question Patrick C. Ma, and also if the comparator arm should be chemo-IO rather than IO alone in this subset.
Patient-level analysis CM77T v CM816, exploring what adj IO adds:
– propensity-matching analysis for EFS
– pCR HR 0.58, nonpCR HR 0.65
– PDL1+ HR 0.86, PDL1- HR 0.51, all favor periop IO
Some 95% CIs cross 1, sample sizes small. But important data.

Ph II NeoCOAST-2 platform trial of neaodjuvant durva+chemo+either monalizumab, oleclumab or dato-dxd:
– incremental gains in pCR with combos
– pCR 25% in PD-L1<1% with dato-dxd
– mildly higher tox
A resurrection of dato-dxd, for this subgroup.

For more updates on WCLC24, visit oncodaily.com
Dr. Jarushka Naidoo is a thoracic oncologist at the Beaumont Hospital Dublin (Ireland), Professor in RCSI and Adjunct Professor of Oncology at Johns Hopkins University. She currently serves as national lung cancer lead for Cancer Trials Ireland. She serves on several international guideline panels including ASCO and SITC (Society of Immunotherapy for Cancer), and is an experienced clinical trialist- having developed and completed several investigator-initiated, industry-funded and cooperative group clinical trials.
She has received a number of grants including a young investigator award from the International Association for the Study of Lung Cancer (IASLC), Lung Cancer Foundation of America (LCFA), and a career development award from the National Institutes of Health (NIH) KL2-program. She is the IASLC Communications Committee Chair.


