At OncoDaily Immuno-Oncology, we bring you the week’s most impactful conversations in cancer immunotherapy—from trial results and breakthrough combinations to expert perspectives shaping the future of IO. This curated highlight reel captures insights from leading oncologists, researchers, and industry voices, offering a pulse check on the science, strategy, and stories driving the field forward.
This Week’s Expert Highlights in Immuno-Oncology
Erman Akkus (Medical Oncology Fellow at Ankara University):
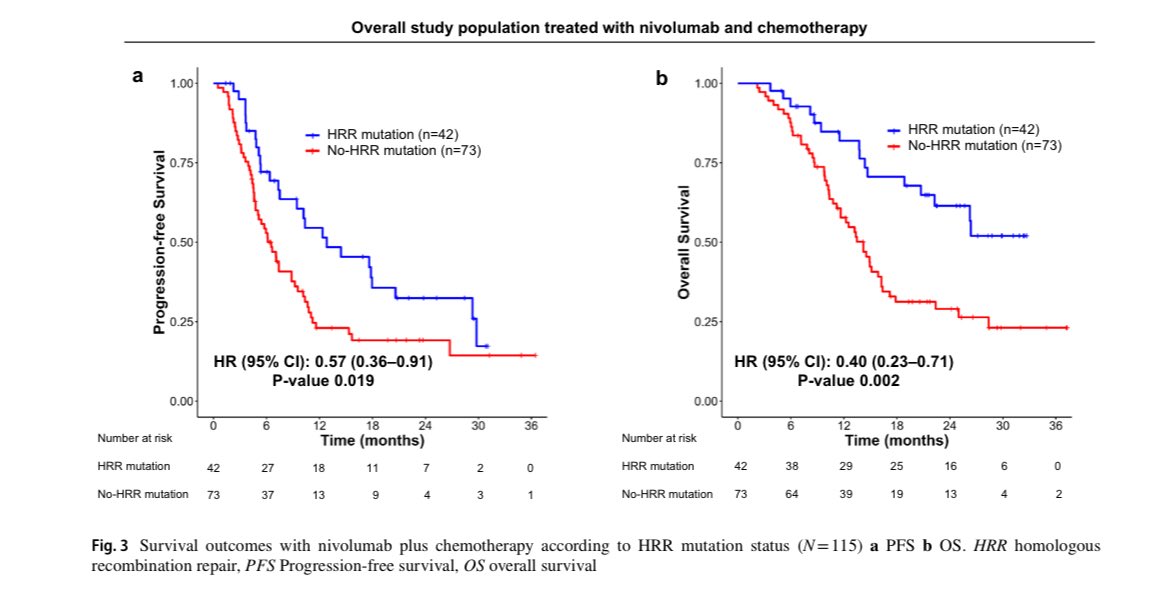
“HRR mutations and first-line nivolumab plus chemotherapy for gastric cancer
- 36.5% had HRR mutations
- Among pts with nivo-ChT HRR vs non-HRR: PFS: 12.8 vs 6.5 mo OS: NR vs 14.2
Nivo-ChT vs ChT: Nivo benefit was apparent for HRR mutant patients but not in non-HRR group.”
You Can Read All Article Here
Stephen V Liu (Chief of Division of Hematology and Oncology at Georgetown Lombardi Comprehensive Cancer Center, Director of Thoracic Oncology and Developmental Therapeutics):
“Had fun talking small cell lung cancer with Dr. Susan Combs Scott – from T Cell Engagers like tarlatamab to ADCs to maintenance lurbinectedin – lots to cover these days. Stay tuned for the final product!”

SITC (Society for Immunotherapy of Cancer):
“Welcome Dr. Julie R. Brahmer of Johns Hopkins Kimmel Cancer Center to the 2025 class of the Academy of Immuno-Oncology. Congratulations on achieving one of SITC’s most prestigious honors! Read Dr. Brahmer’s bio and learn more here:”

Spencer Knight (Principal Business Consultant of Cell and Gene Therapy):
“MS Patient Walks Again After 60 Days
Jan Janisch-Hanzlik, 49, is the first in the world to receive a groundbreaking allogeneic CAR T-cell therapy for multiple sclerosis at Nebraska Medicine. This innovative treatment uses genetically engineered donor T-cells to target the immune cells causing MS damage.
Highlights:
- After only 60 days, Jan was able to walk again – a remarkable recovery.
- The therapy is “off-the-shelf,” meaning it’s readily available without needing to custom-make cells for each patient.
- Previously successful in treating blood cancers and lupus, this approach now offers new hope for MS patients worldwide.Jan shares, “I’m excited for the entire MS community – this could change lives.”Amazing to see Jan’s excitement after her MS Diagnosis 7 years ago. Dr. Rana Zabad, MS specialist, and Dr. Matt Lunning, CAR T-cell therapy expert, praise Jan’s courage and anticipate this could open doors to new treatment options for many.
Huge thanks to Dr.Zabad, Dr. Lunning, Nebraska Medicine, and all involved.:”

Nieves Martinez Lago (Specialist in the Field (FEA) of Medical Oncology at University Hospital Complex of Santiago de Compostela):
“ Immunotherapy + periop FLOT improves EFS in resectable GEAC
Meta-analysis of KN-585 & MATTERHORN using reconstructed KM curves
- FLOT+IO EFS vs FLOT or cis/5FU+IO
- No EFS diff: DURVA vs PEM
- FLOT = preferred periop CT backbone for IO “

Get the Full Insight Here
ESMO (European Society for Medical Oncology):
” ESMO Immuno 25: Aurélien Marabelle, Scientific Co-Chair, invites you to present your research to an international audience of top-tier scientists, clinicians, and industry experts.
Deadline: 30 September 2025
We welcome submissions on a wide range of topics, including:
- Biomarker development
- Cell therapy/Immune engineering
- Clinical practice
- Methodological, financial and regulatory aspects
- Therapeutic development
- Tumour biology and tumour microenvironment
- Miscellaneous “

Raffaele Colombo (Associate Director of Medicinal Chemistry at Zymeworks):
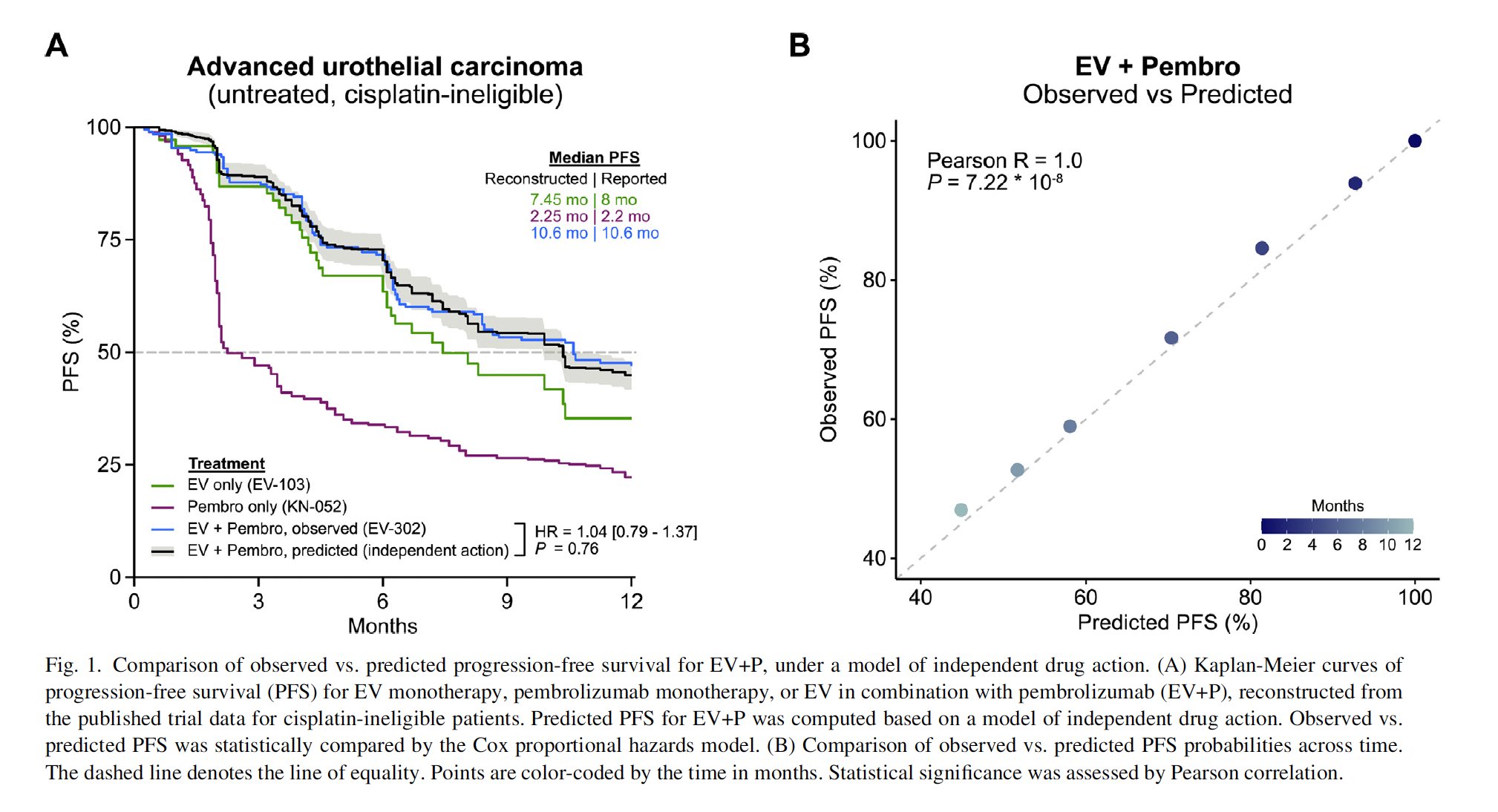
“Is there a synergistic effect between enfortumab vedotin (EV) and pembrolizumab (P) for untreated advanced urothelial carcinoma in clinical setting?
This analysis suggests no!
EV+P seems to follow a an independent drug action model with no evidence of synergy”
Yüksel Ürün (Medical Oncology professor at Ankara University School of Medicine):
“Congrats to all who made this possible. This trial showed 53% pCR and 64% 2‑yr EFS with neoadjuvant chemo‑immunotherapy in non-urothelial MIBC subtypes. Time for collaboration and more studies in this neglected population.”
Continue Reading the Full Feature Here
Diego Díaz García (Medical Oncologist / CEO / Founder at CánCare – Advanced Specialty in Oncology):
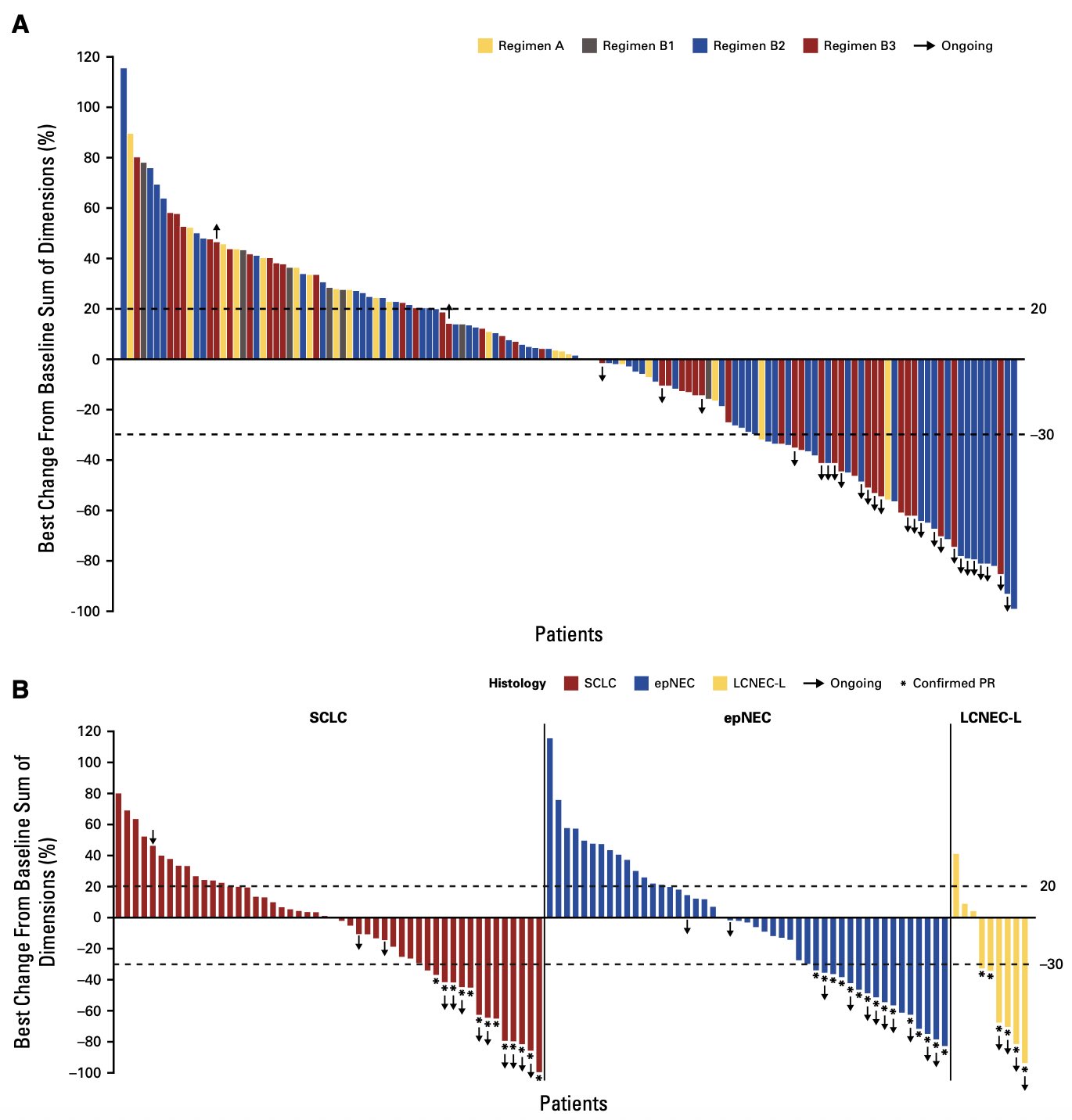
” DLL3/CD3 T-cell engager obrixtamig (BI 764532) in SCLC & neuroendocrine carcinomas (ph I):
- n=168, heavily pretreated (72% ≥2L, 51% prior PD-1/L1)
- ORR: 23% overall; B2/B3 regimens ≥90 mg/kg: ORR 28%
- SCLC 21% • epNEC 27% • LCNEC-L 70%
- Median DoR: 8.5 mo (6-mo DoR rate 70%)
- CRS: 57% any grade, 3% ≥G3, early/reversible “
Read the Full Article Here.
Marco Donia (Research Group Leader of the TIL group at CCIT (Center for Cancer Immune Therapy) and Professor at the University of Copenhagen):
” In‑vivo BCMA CAR T‑cell therapy for relapsed/refractory multiple myeloma (The Lancet, Xu et al., July 2025)
First‑in‑human, in‑vivo BCMA CAR‑T therapy, delivered via a lentiviral vector that programs a patient’s own T cells inside the body, skipping leukapheresis, conditioning chemo, and manufacturing delays.
Key points
- Novel delivery: CAR‑T cells generated in vivo via vector injection, no external manufacturing required
- Efficacy: 4/4 responders
- Safety: Manageable Cytokine release syndrome
Clinical implications, This approach could transform CAR‑T accessibility Larger trials, follow-up for long‑term risks, and comparative performance versus standard CAR‑T or bispecific therapies needed
Take‑home message- Early data, but potentially big step forward in CAR-T therapy.More about the challenges of in vivo CAR-T: ”