The ASCO Genitourinary Cancers Symposium (ASCOGU25) is an annual event organized by the American Society of Clinical Oncology (ASCO), focusing on the latest advancements in genitourinary (GU) cancer research and treatment.
The 2025 symposium, which is taking place from February 13th to 15th, will feature high-impact science, multidisciplinary expertise, and evidence-based practices in GU oncology.

Our team at OncoDaily has selected a few posts from this event that you should not miss!
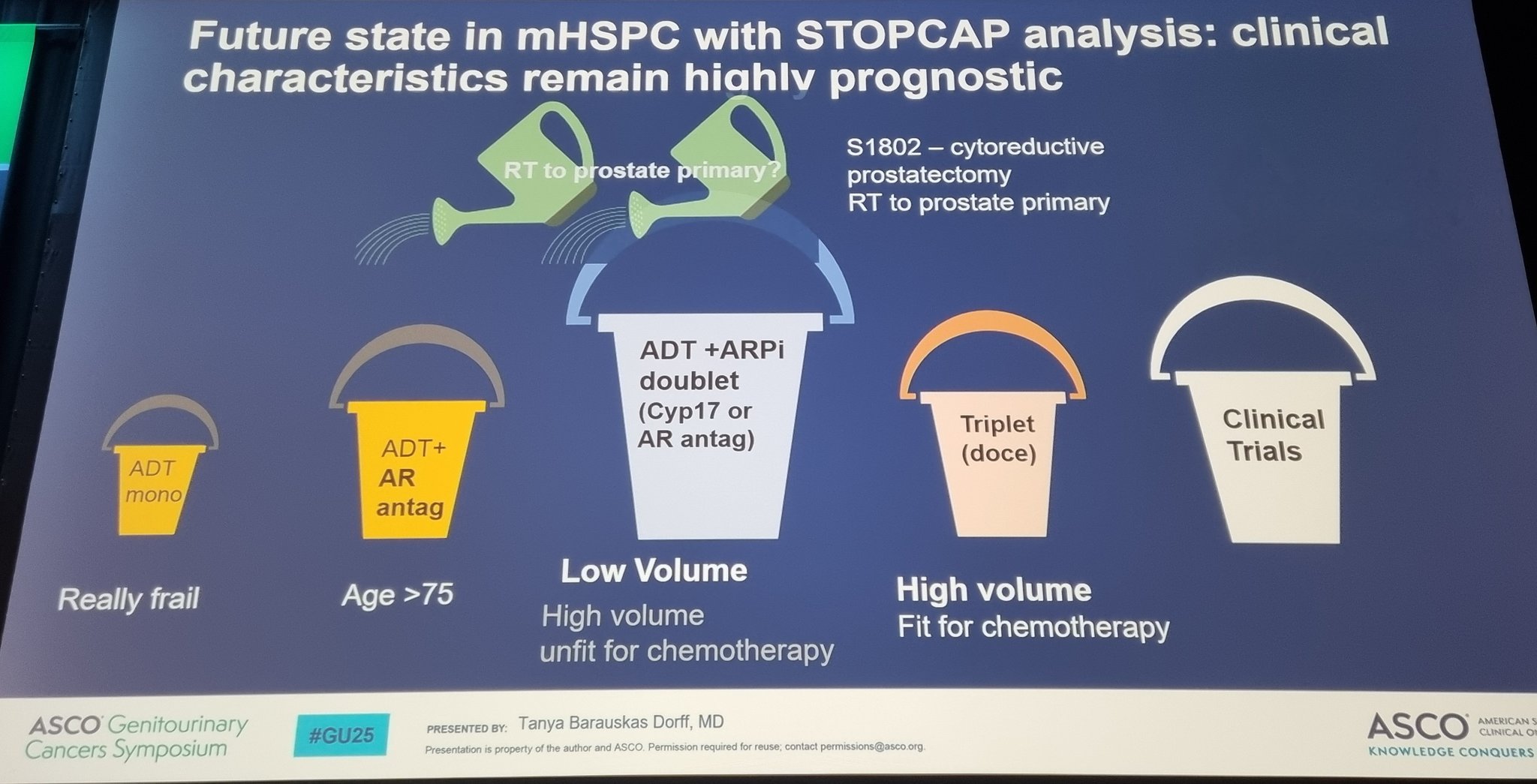
“Beautiful synthesis of complex datasets at ASCOGU25 from Tanya Dorff, leader of our GU team at City of Hope! The “buckets” she notes truly mirror our experience in the clinics and love the aspiration towards a more biologically driven approach. Fellows: So much to learn from her presentation style!”
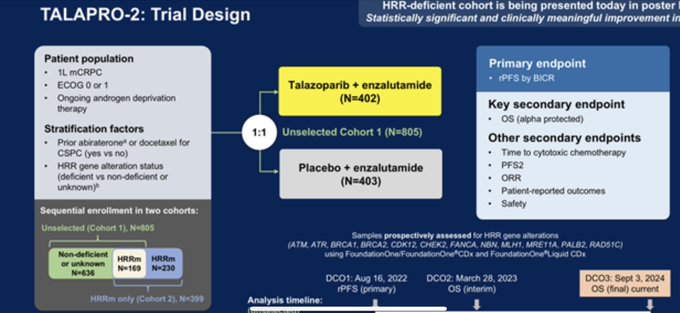
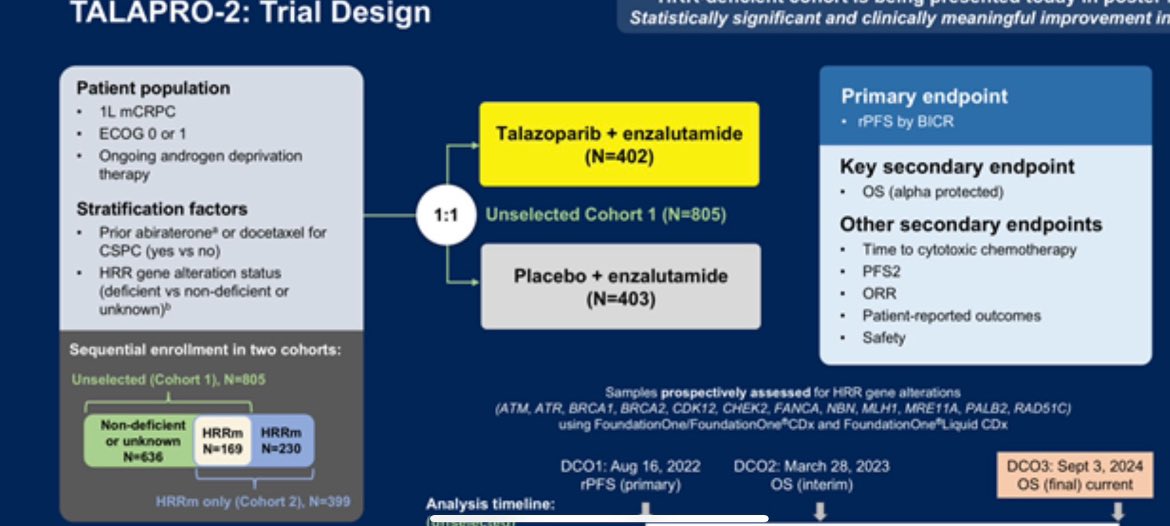
“ASCOGU25 spectacular presentation by Neeraj Agarwal on the practice-changing data from TALAPRO-2 trial enza and talasoparib vs enza 1LmCRPC. OS 8.8 months significantly longer in all comers. rPFS 13.6 months longer with combo in all comers. No new safety signals.”
“A heartfelt celebration of life for Felix Feng, honoring his incredible legacy and dedication to cancer research. In his memory, we will continue the ‘Felix Feng Endowed Conquer Cancer YIA in Prostate Cancer Research,’ ensuring his impact continues for future generations.”

“TALAPR02 looks at Talazoparib/enza vs enza in 1st line CRPC . It has significant OS in ITT HR=0.796. It’s currently used in HRR deficient. Subset analysis and wider data still support the HRR biomarker OS (HRR deficient HR -0.54, HRR non-deficient/NA 0.87), but ITT OS is positive.”
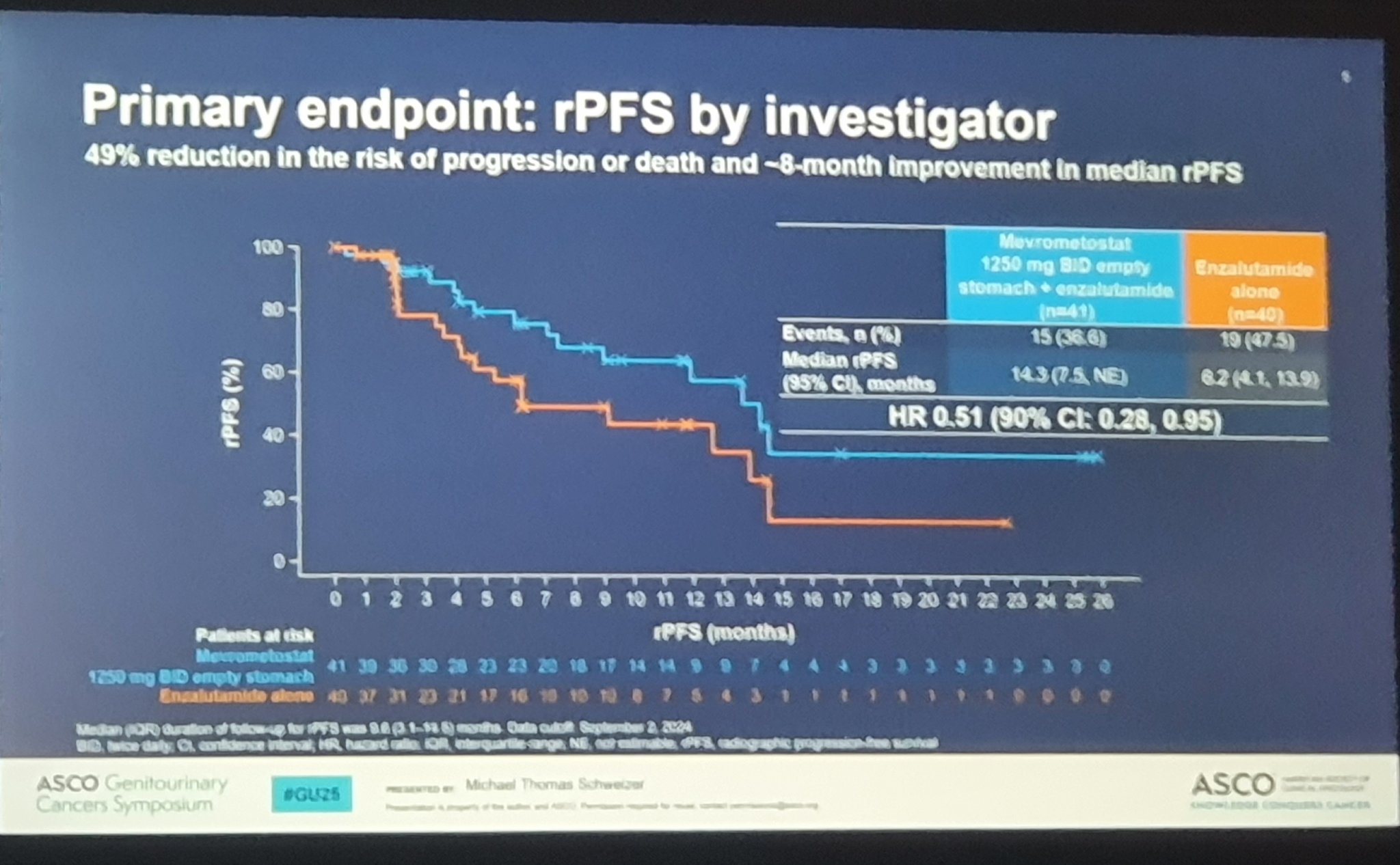
“Abstract LBA138
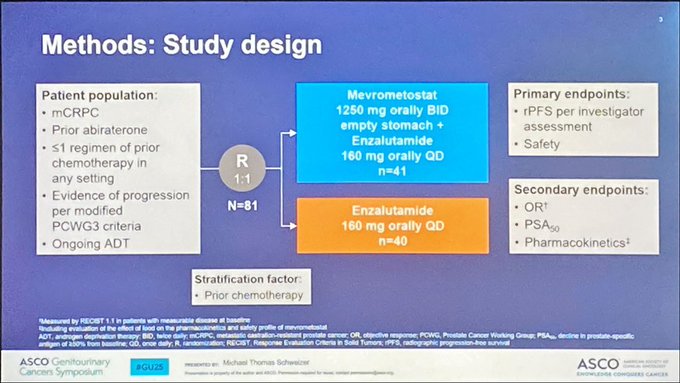
Mevrometostat (EZH2i) and Enzalutamide in mCRPC: Dose-Expansion Results
Key Results:
- rPFS: 14.3 vs. 6.2 months (HR 0.51; 90% CI 0.28-0.95)
- ORR (measurable disease): 26.7% vs. 14.3%
- PSA50: 34.1% vs. 15.4%
- Grade ≥3 TEAEs: 53.7% (M+E) vs. 42.5% (E) (most common: diarrhea, neutropenia, sepsis)
- Optimized dosing: M 875 mg plus food equals improved safety vs. 1250 mg fasting
- Conclusion: Mevrometostat + Enzalutamide shows promising efficacy over Enzalutamide alone in mCRPC, with a manageable AE profile. Further studies warranted.
“Mike Schweizer with mevrometostat and enzalutamide vs enza comparison for prostate cancer — better PFS and robust PSA declines. We had patients who benefitted on trial! Look forward to the phase 3s.”
“One of the takeaway slides from this ASCOGU25 about current and future management of mCSPC from a terrific presentation by Tanya Dorff.”
“Timely and elegant ASCOGU25 Keynote lecture delivered by Bill Dahut. In our ever-evolving oncology field, innovation must be accessible to everyone – AI will have a transformative role in advancing patient-centred care. Thank you for highlighting our work!”
“Congrats to Hoosier Cancer GU co-chairs,RanaMcKay and Shilpa Gupta. Leading a room filled to the brim with top investigators at ASCOGU25, it’s impressive to see the breadth of trials their group is doing.”
“Mevrometostat (PF-06821497), an enhancer of zeste homolog 2 (EZH2) inhibitor, in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer (mCRPC): A randomized dose-expansion study.”
“Great to see our amazing fellow Hershey Dudipala presenting data on our molecular profiling of intraductal prostate cancer at ASCOGU25! Associated with NEPC signature, DLL3 expression and worse molecular features.”

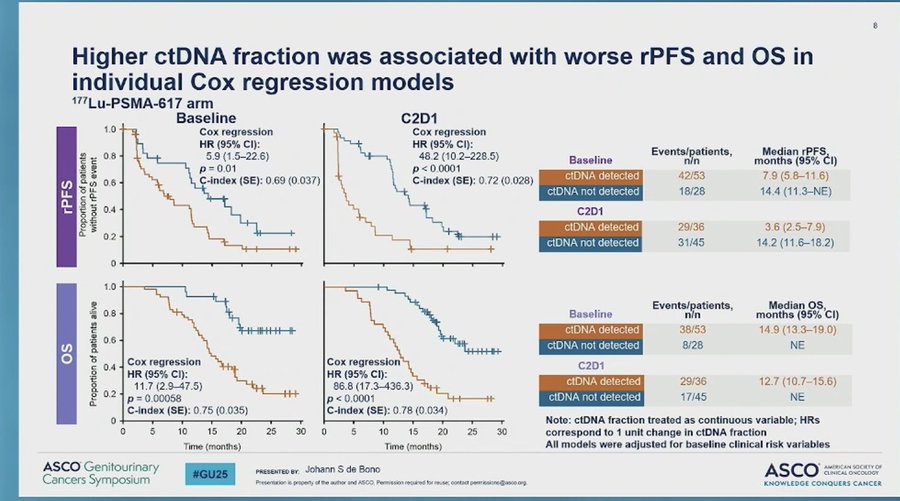
“Check out the data from PSMAfore biomarker analysis: Huge prognostic significance in baseline and 6-week on treatment ctDNA levels and outcomes. Adds to PSA 50 response as well. Could guide future management in this earlier disease setting.”
“Prostate Cancer Rapid Oral Abstracts ASCOGU25:
Dr Michael Schweizer presenting Ab138 “Mevrometostat (PF-06821497), an (EZH2) inhibitor, in combination with ENZA in patients with (mCRPC): A randomized dose-expansion study.”
- N = 81
- mCRPC prior ABI, <1 chemo (~45%), PD per PCWG3
- M + ENZA (n =41)
- ENZA (n =40)
- rPFS: M+E 14.3 months vs E 6.2 months (0.51; 90% CI 0.28, 0.95)
- ORR: M+E 26% vs E 14%
- PSA50: M+E 34%% vs E 15%
- TEAEs M+E – diarrhea (78%) – decreased appetite (58.5%) – dysgeusia (58.5%)
- Clinical implications: – M+E shows improved outcomes vs E in pts with mCRPC, with a manageable AE profile”
“Great seeing colleagues. I still remember interviewing with Jeff Michalski for residency (car broke down in St Louis). Also applying for a job out of residency with Howard Sandler…and to those in residency…he didn’t even interview me.
He chose wisely as he hired my coresident Zumsteg who is absolutely phenomenal (I would have recruited him over me too). Met Jonathan Tward early on and great collaborator. Doesn’t seem that long ago. Time flies.”
“I am so proud of these two fellows of mine who received the ASCO merit award at ASCOGU25. Tutorship meaning is to improve young careers, allowing new experiences and sharing knowledge.”
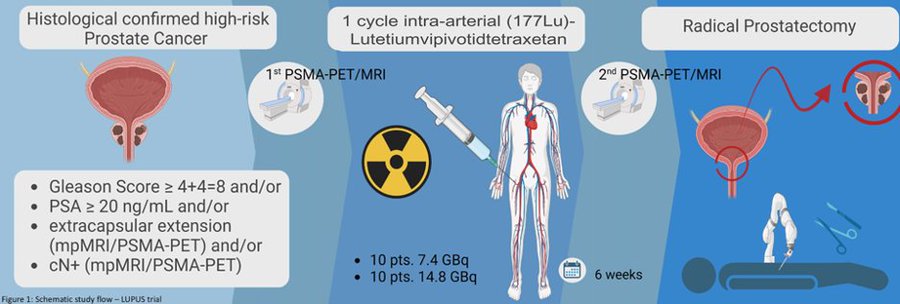
“ASCOGU25 TiP:
LUPUS: Phase 1/2 trial of 1 cycle of intra-arterial of (177Lu) Lutetium vipivotide tetraxetan in HR PCa patients prior to RP. Primary endpoint: AEs + days of delay of RP due to AEs. Secondary endpoint: PSA response, pCR, MRD, PET/MRI changes, PROs, QoL.”
“The final OS results from cohort 1 of TALAPRO-2 trial are being presented by Neeraj Agarwal at the 2025 ASCOGU. It has significant OS in ITT HR=0.796.”
“Day 1 of ASCOGU25- PCa updates to come including:
- ALAPRO2 role of PARPi in unselected population?
- STOPCAP who benefits more from ADT/ARPI vs ADT/docetaxel?
- PSMAfore ctDNA analysis
- ENZAP enza/Lu-617 OS analysis
- Case-based discussions on BCR and oligometastatic PCa”
For more updates on this conference, visit oncodaily.com