The European Lung Cancer Congress (ELCC 2025) takes place in Paris from March 26-29, 2025, bringing together leading experts in thoracic oncology. Designed for medical oncologists, radiation oncologists, thoracic surgeons, pneumologists, interventional radiologists, and pathologists, ELCC offers a comprehensive program focused on advancing lung cancer research and improving clinical practice.
Many physicians and organizations have shared posts from Day 3 of ELCC 2025.
“Updated OS from the LAURA study shows the trend for OS benefit with osimertinib over PLB continuation after cCRT: IA 54 vs NR, HR 0.81, ns (20% events); Updated A 58.8 vs 54 months, HR 0.67, (31% events). Subsequent 3G-TKI 80% in the placebo group!”
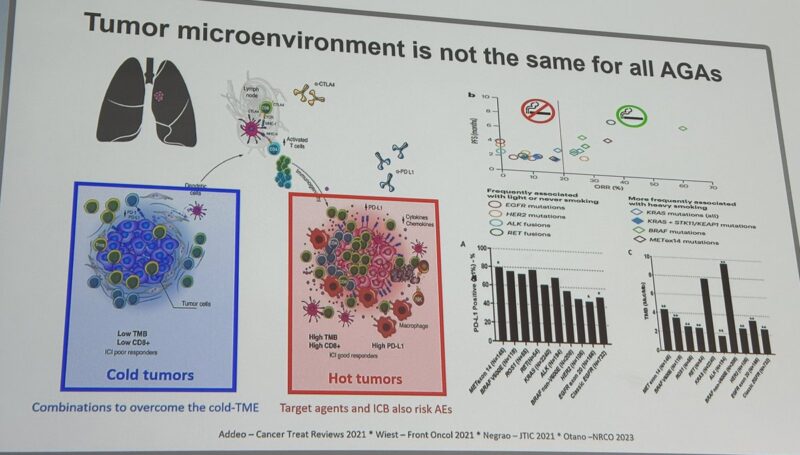
“Great presentation by Jordi Remon at ELCC25 on ‘hot’ topics in advanced NSCLC, dissecting the role of ICB in immunologically ‘cold’ and ‘hot’ mutations. And thanks for Sharing one of our papers.”
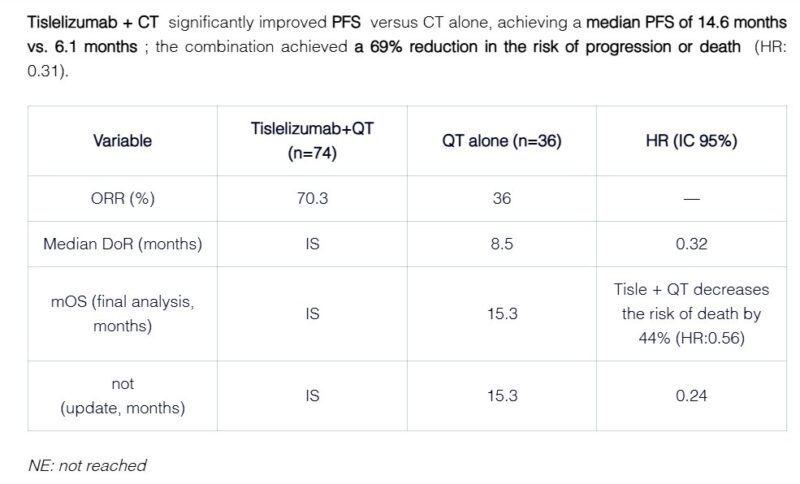
“Tislelizumab + QT: A New Hope in NSCLC.
At ELCC2025, Dr. Yan Yu presented an analysis from the Phase III RATIONALE-304 study, focused on this subpopulation with high potential for immunotherapeutic response.
Objective: Evaluate the efficacy of tislelizumab combined with chemotherapy (QT) in patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC).
Inclusion Criteria:
- Diagnosis of advanced or metastatic non-squamous NSCLC
- PD-L1 expression ≥50% in tumor cells
- No EGFR or ALK mutations
Key Findings: Progression-Free Survival (PFS): Median PFS of 14.6 months with tislelizumab + QT vs. 4.6 months with QT alone.
Objective Response Rate (ORR): Higher response rates with the combination of tislelizumab and QT.
Safety: Most common grade ≥3 adverse events: neutropenia and leukopenia, primarily associated with QT.
Conclusion: The combination of tislelizumab and chemotherapy significantly improves PFS and response rates in PD-L1 high-expression NSCLC, offering a promising therapeutic option.”
“MAPS2 bulk RNAseq stratification suggests that the time has come for a better prediction of IO response beyond histology. Maybe DSP and single cell RNS seq could provide more insights?”
“So after 10 years of first approval of IV pembrolizumab in NSCLC, we now have data for subcutaneous pembrolizumab vs IV pembrolizumab.
It has similar PFS and toxicity. Good for patients.
Why does it take 10 years for subcutaneous data to come? Any idea?”
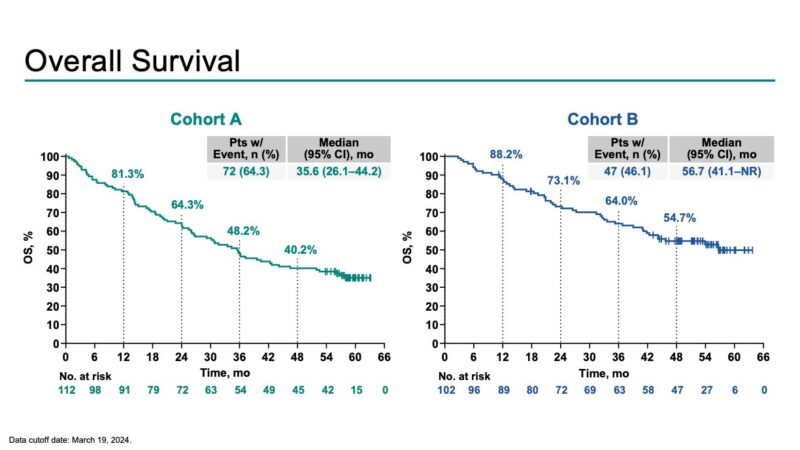
“Pembro + cCRT in unresectable LA NSCLC – Final KN-799: ORR >70% (across histologies)! mPFS 29 and 45 months, mOS 36 and 57 months (cohort A NSCLC, B NSQ).
Sharp discussion by S. Ramella.
UNKNOWNS: Impact of RT modality and immune response.”
“More than 3 years follow up from the VISION study (METex14 mutated advanced NSCLC) treated with tepotinib: 57.9% ORR, 12.6 m PFS in treatment-naive patients. And still not reimbursed in France.”

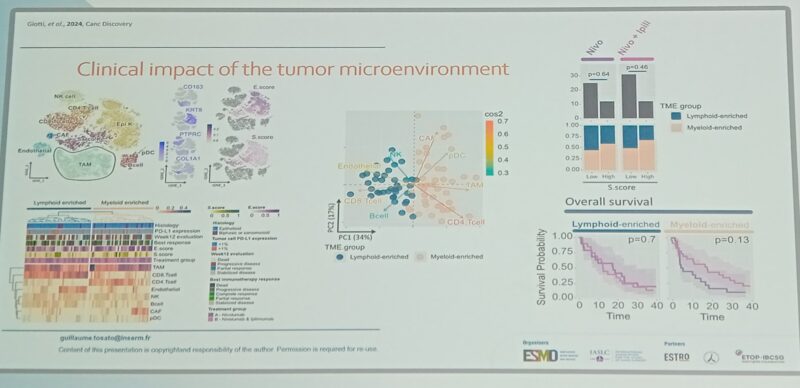
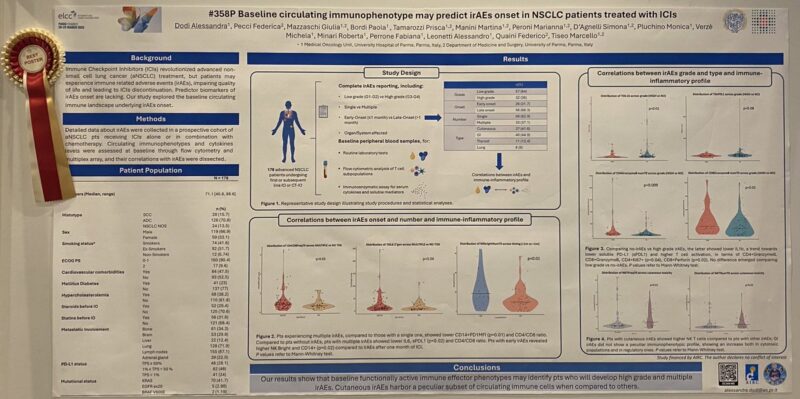
“irAEs negatively impact QoL and may lead to early ICIs interruption in NSCLC.
A baseline cytotoxic and activated circulating immunophenotype may anticipate the onset of high-grade and multiple irAEs, allowing clinicians to set up prevention strategies.”
“Excited to share new clinical data at ELCC 2025!
Today, PDC*line Pharma is proud to present new results from our ongoing clinical development program at the European Lung Cancer Congress 2025 (ELCC2025), during the poster session — in the presence of our Principal Investigator, Prof. Johan Vansteenkiste.
We are sharing key findings on PDC*lung01, our innovative, ‘off-the-shelf’ therapeutic cancer vaccine for non-small cell lung cancer (NSCLC).
Poster Title: Primary analysis of safety, efficacy and immunogenicity of the therapeutic cancer vaccine PDClung01 with or without pembrolizumab in NSCLC: A multicenter phase I/II study.
Key highlights from the study:
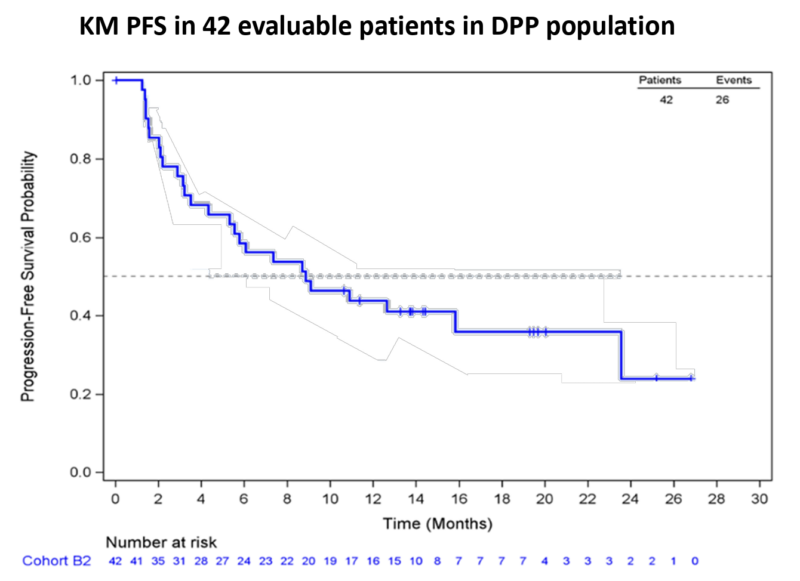
- In Cohort B2, the confirmed Overall Response Rate (ORR) of 55% and the median Progression-Free Survival (mPFS) of 8.87 months represent an improvement of 16% in ORR and 2.4 months in mPFS compared to the Keynote-042 PD-L1 ≥50% population.
- PDC*lung01 combined with pembrolizumab provides meaningful clinical activity in untreated PD-L1 ≥50% stage IV NSCLC, with a mild safety profile, and a confirmed correlation between the immune response intensity and the PFS.
We look forward to engaging with fellow researchers, clinicians, and industry leaders at ELCC 2025 to discuss how PDC*lung01 may help shape the future of immuno-oncology.”
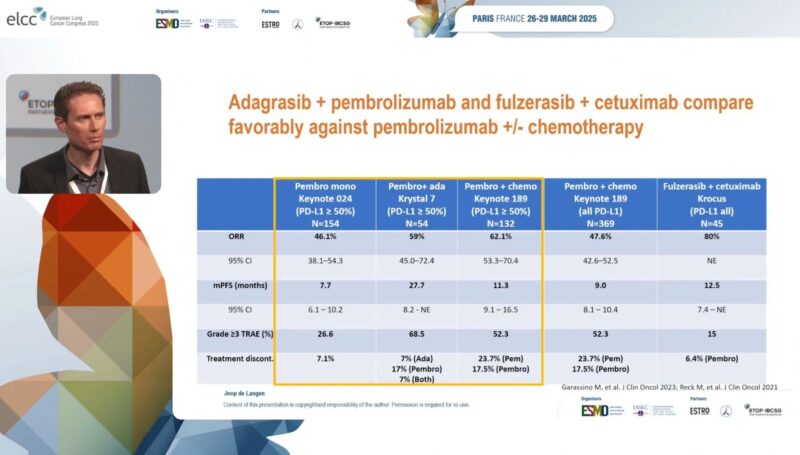
“Can we improve outcomes of KRAS mutated advanced NSCLC? What’s the new data?
Check the slide to compare the results of adagrasib plus pembrolizumab and Fulzerasib plus Cetuximab with the current SOC. Money slide!
Daraxonrasib. New first-in-class pan RAS inhibitor. Compared with Adagrasib and Sotorasib in NSCLC.”
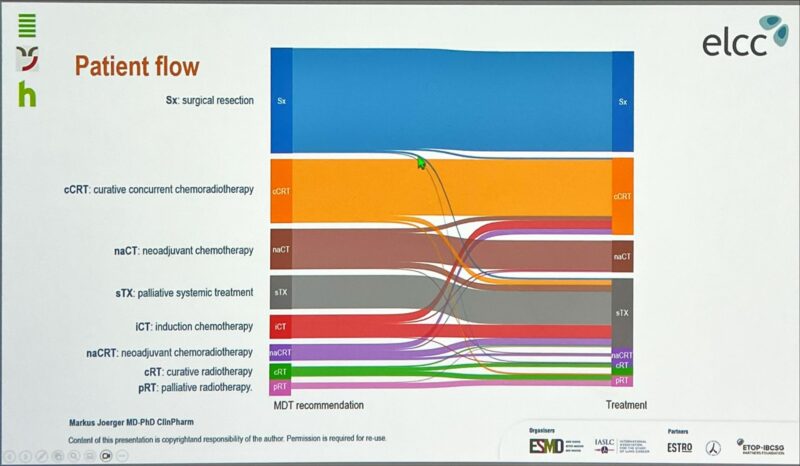
“Can MDT decisions have an impact on patients with stage III NSCLC?
Non-adherence was associated with worse outcomes.
Elderly and frail patients are at higher risks of non-adherence and merits special consideration.”
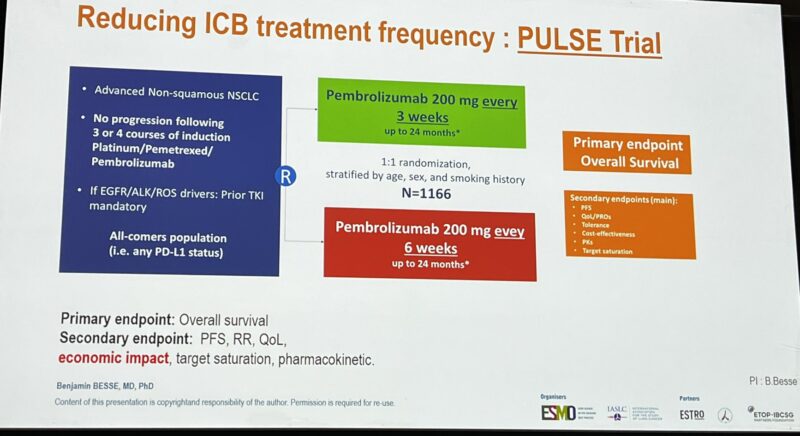
“De-escalation clinical trials in NSCLC
Impassioned presentation of the key trials, that can save patients’ time, and toxicity, and ensure healthcare system sustainability:
- PULSE (France)
- Osi-Save (Netherlands)
- REFINE-Lung (United Kingdom)
Benjamin Besse is the best in the business.”
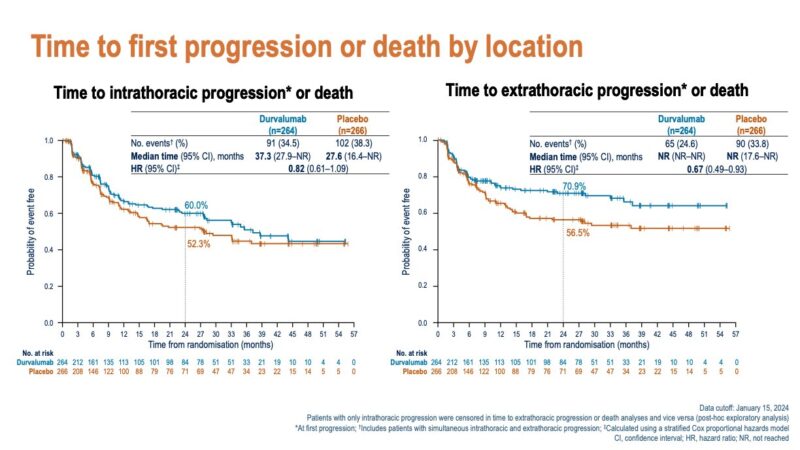
“Patterns of disease progression with durvalumab after concurrent cCRT in LE-SCLC in ADRIATIC: time to CNS PD prolonged and lower rates of CNS mets with durvalumab irrespective of PCI use.
Nicely presented by Dr. Suresh Senan.”
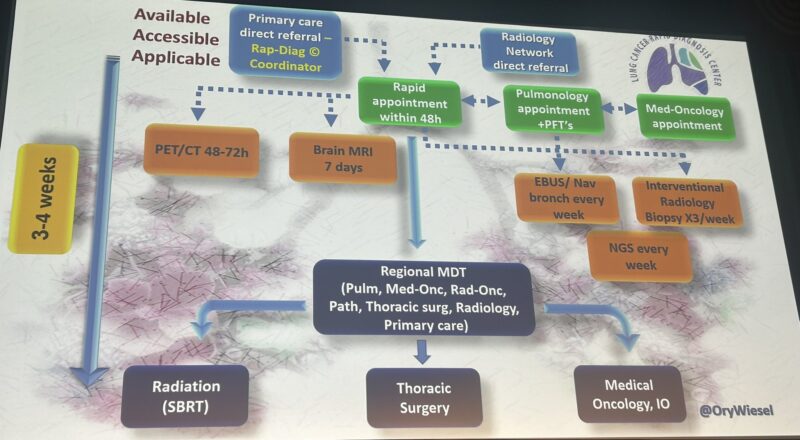
“Interesting workflow giving rapid and equal access to lung cancer patients (regardless of origin/religion/social status) in Israel by Ory Wiesel at ELCC25.
Medicine is often better than politics.”