A fungal natural product first isolated in 1970, (+)-verticillin A, has attracted scientific attention for decades because compounds in its chemical family have shown anticancer activity in preclinical models. The problem has always been access: verticillin A occurs only in trace amounts in nature and is challenging to purify at scale.
In late 2025, a collaborative team spanning MIT and Dana-Farber/Harvard Medical School reported the first total synthesis of (+)-verticillin A, creating a reliable lab route to produce the molecule on demand and to design analogs for deeper biological testing.
From Natural Product Curiosity To Synthetic Priority
Verticillin A belongs to a class of fungal metabolites that fungi may use for defense. Although its structure was known for decades, the compound’s scarcity limited rigorous study, including systematic comparisons across cancer models and the type of medicinal chemistry optimization typically needed to move a “hit” toward a drug candidate.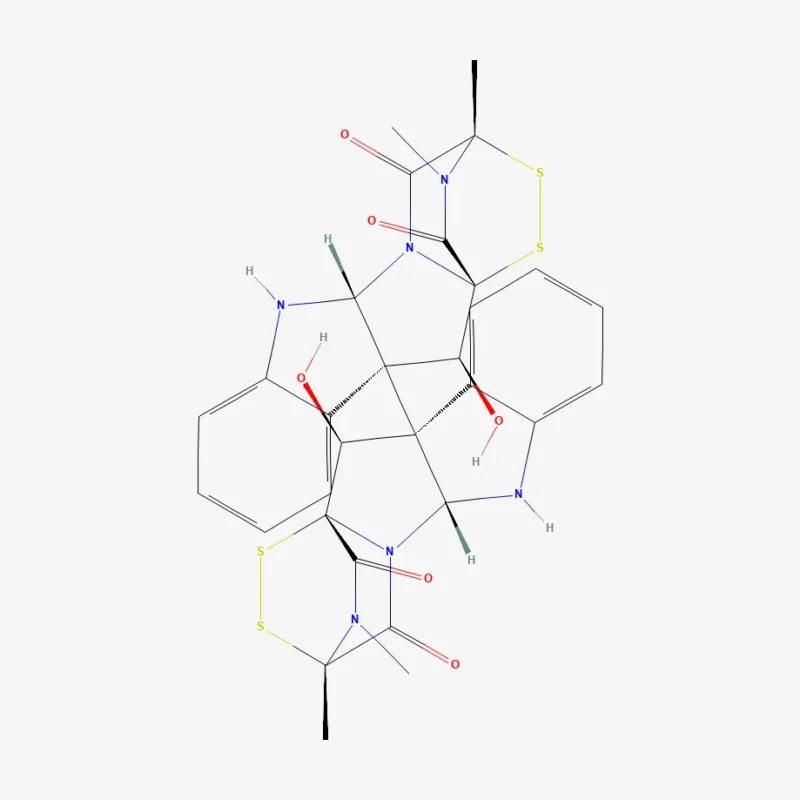
Why Verticillin A Resisted Synthesis For Decades
Chemically, verticillin A is a dimer two highly similar halves joined together packed with stereochemical complexity and reactive sulfur-containing features. The MIT team notes that even small structural differences from related molecules can sharply narrow the “safe” window for chemical transformations, increasing fragility during multi-step synthesis.
The new paper describes how earlier strategies could efficiently build related scaffolds, yet still failed to deliver verticillin A’s correct three-dimensional arrangement an issue that matters because the biological activity of many natural products depends on precise stereochemistry.
A 16-Step Total Synthesis Opens The Supply Bottleneck
The researchers report a 16-step synthesis (from a defined starting material) that successfully constructs (+)-verticillin A more than five decades after its original isolation.
A central lesson, described in both the institutional report and the paper, is sequence control: the team re-ordered key bond-forming events and used protective strategies to prevent sensitive sulfur features from decomposing before the final structure was assembled.
Beyond The Natural Product: Making Designed Variants
One of the most practical outcomes of a total synthesis is not only replicating the natural molecule, but also enabling structure–activity relationship work systematically changing parts of the molecule to improve stability, potency, or selectivity. The authors report generating and evaluating verticillin-related derivatives, including versions engineered for greater stability.
Early Anticancer Signals In Diffuse Midline Glioma Cell Models
The biological focus in this report includes diffuse midline glioma (DMG), a rare and aggressive pediatric brain tumor with limited treatment options. In cell-based studies, verticillin A and selected derivatives showed antiproliferative (cell growth–inhibiting) activity in DMG models, supporting further investigation rather than serving as evidence of clinical efficacy.
Notably, the team observed that susceptibility in certain DMG lines tracked with molecular context, including EZHIPexpression and low H3K27me3 levels features linked to altered chromatin regulation in subsets of pediatric gliomas.
Vorasidenib Approved in the UK: New Treatment for IDH1/2-Mutant Low-Grade Gliomas
Mechanistic Clues: Target Engagement With EZHIP
A key mechanistic thread centers on EZHIP, a protein known to interfere with EZH2 function in relevant tumor contexts. In the open-access report, the authors describe experiments consistent with direct engagement of EZHIP by a lead verticillin-derived compound using a cellular thermal shift assay approach, alongside downstream molecular changes consistent with re-activating EZH2-associated methylation marks.
At the same time, the paper emphasizes that context-dependent mechanisms may exist, including cytotoxic effects in DMG models that do not strongly express EZHIP underscoring why broader profiling and careful target validation remain essential before translational conclusions are drawn.
What Changes Now For Drug Discovery
The leap from “interesting natural product” to “therapeutic candidate” usually depends on three capabilities: dependable supply, the ability to create analogs, and mechanistic clarity. This work directly advances the first two and begins to address the third.
Important next steps many of which the investigators highlight include validating mechanism across models, optimizing properties relevant to medicines (including stability and selectivity), and expanding testing into in vivosystems such as animal models. For brain tumors in particular, questions about delivery and tolerability become decisive well before any human studies can be justified.
Written by Nare Hovhannisyan, MD



