Tom Powles, Professor at the University of London, shared a post on X:
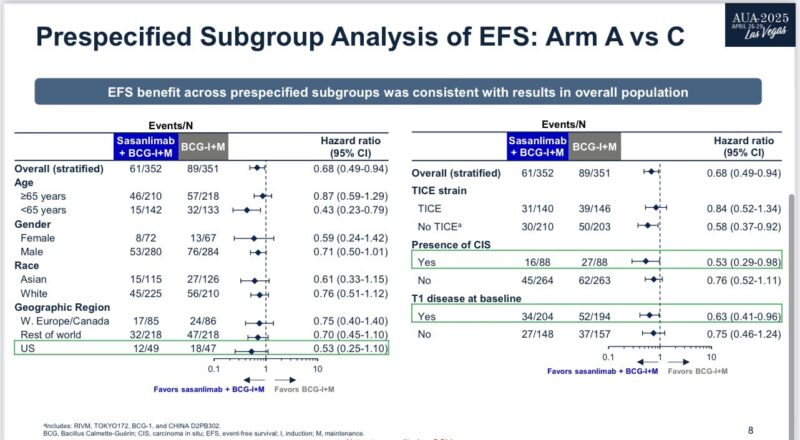
“BCG plus sasanlimab vs BCG = increase EFS by 32% (HR=0.68, min FU 3 years, n=1050).
Decrease recurrent high risk NMIBC was the predominant feature. No OS signal. Enrichment in relevant subgroups such as CIS (HR=0.53). Tox: 29% vs 6% Grade 3-4 TRAEs is part of risk/benefit decision.
2 yrs of subcut sasanlimab = ~10% chance of problematic/life changing immune tox. EFS includes serious cancer risk (MIBC, advanced disease, UC death), but also HG NMIBC. An unselected approach will mean over treatment for some. Delivery in urology clinics requires planning.”
Tom Powles shared results from the Phase III CREST trial, highlighting a 32% improvement in event-free survival (EFS) with BCG plus sasanlimab compared to BCG alone in patients with high-risk non-muscle invasive bladder cancer (NMIBC). The trial showed no overall survival (OS) signal, but notable benefit in subgroups such as carcinoma in situ (CIS). Grade 3–4 treatment-related adverse events (TRAEs) occurred in 29% of patients receiving combination therapy versus 6% with BCG alone, informing the risk-benefit assessment.
More posts featuring Tom Powles.