At ASCO 2025, Dr. Benjamin P. Levy and an international team of investigators presented new findings from a randomized subset of the Phase II SAVANNAH trial, evaluating the selective MET inhibitor savolitinib (savo) in combination with osimertinib (osi) in patients with EGFR-mutated (EGFRm) advanced non-small cell lung cancer (NSCLC) and evidence of MET-driven resistance following progression on first-line osimertinib. These data provide critical insight into the contribution of MET inhibition in overcoming resistance and show early promise in controlling CNS disease.
What Is the SAVANNAH Trial?
The SAVANNAH trial (NCT03778229) is a Phase II clinical study designed to address a critical challenge in treating advanced non-small cell lung cancer (NSCLC) with EGFR mutations—resistance that develops due to activation of the MET pathway. This trial specifically explores whether combining two targeted therapies—savolitinib, a highly selective MET tyrosine kinase inhibitor (MET-TKI), and osimertinib, a third-generation EGFR inhibitor—can help overcome this resistance in patients who have progressed after first-line osimertinib treatment.
In this randomized, double-blind trial, patients were eligible if their tumors showed signs of MET-driven resistance following progression on osimertinib. Two key biomarkers were used to identify MET activation: MET overexpression, which was defined as IHC 3+ staining intensity in 90% or more of tumor cells, and MET amplification, determined by the presence of at least 10 MET gene copies on FISH testing.
Participants were randomized in a 2:1 ratio to receive either savolitinib (300 mg twice daily) plus osimertinib (80 mg once daily) or savolitinib with a placebo. Notably, the trial included patients with brain metastases, provided those metastases were either stable or previously treated. Stratifying randomization based on the presence of brain metastases allowed researchers to more accurately assess how well the treatment worked in the central nervous system (CNS).
The study’s primary goals were to evaluate how effectively the combination could shrink tumors (objective response rate or ORR), how long the response lasted (duration of response or DoR), and how long patients lived without disease progression (progression-free survival or PFS). These outcomes were assessed both by independent central reviewers and the patients’ investigators. Additionally, the trial examined how the treatment affected brain metastases by evaluating CNS-specific PFS and tracking the development of any new CNS lesions.
In essence, the SAVANNAH trial offers a focused strategy to address a known mechanism of resistance in EGFR-mutated NSCLC and provides valuable insight into both systemic and CNS-specific outcomes with this dual-targeted approach.
Key Findings and Efficacy Outcomes
In the SAVANNAH trial, patients enrolled in the two treatment arms were generally well balanced in terms of baseline characteristics. The median age was 67 years in the savolitinib plus osimertinib group and 65 years in the savolitinib plus placebo group. A greater proportion of patients in the combination arm were female (73% vs 64%) and White (73% vs 52%).
The combination of savolitinib and osimertinib demonstrated markedly superior clinical activity compared to savolitinib plus placebo across all measured outcomes. The objective response rate (ORR) as assessed by blinded independent central review (BICR) was 58% in the combination group, compared to just 16% in the placebo group. Investigator-assessed ORR was also higher with the combination—54% versus 24%, respectively.
In terms of duration of response (DoR), patients receiving savolitinib and osimertinib had a median DoR of 11.8 months, whereas those on savolitinib with placebo had a shorter median response duration of 4.5 months.
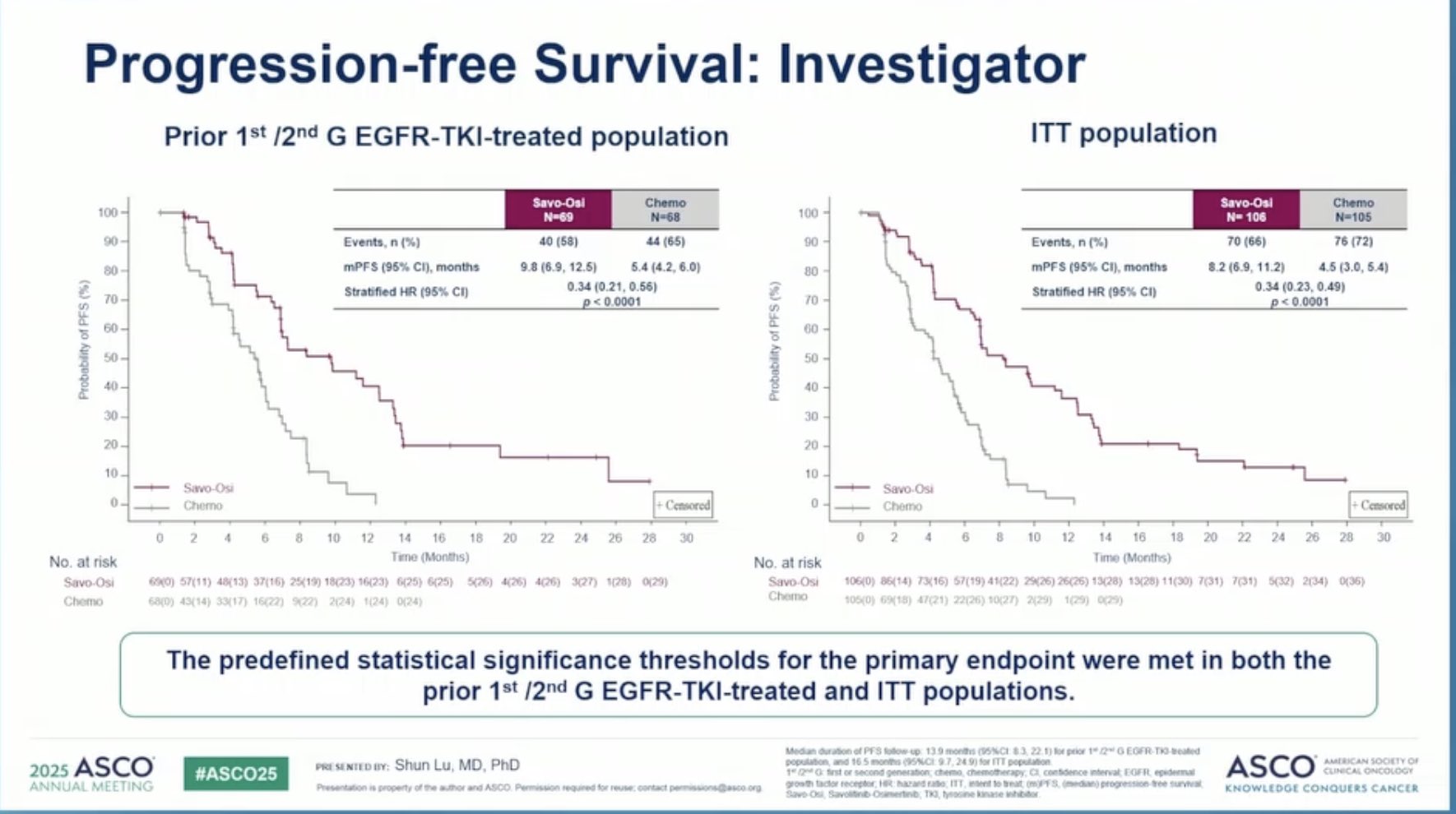
Progression-free survival (PFS) also favored the combination arm. Median PFS by BICR was 8.3 months with savolitinib plus osimertinib, compared to 3.6 months with savolitinib plus placebo. Investigator-assessed PFS showed a similar pattern, with a median of 7.6 months in the combination arm and only 2.7 months in the placebo arm.
Taken together, these results clearly underscore the added value of dual EGFR and MET inhibition, especially in patients whose tumors harbor MET-driven resistance mechanisms. The combination of savolitinib and osimertinib not only improved response rates but also prolonged both response duration and disease control, reinforcing its potential as a targeted strategy in this biomarker-selected population.
CNS Outcomes
The study also explored CNS-specific efficacy:
-
Among patients with baseline brain metastases, CNS PFS events occurred in 36% of those treated with savo + osimertinib (5 of 14), compared to 50% in the placebo group (2 of 4).
-
Among patients without baseline brain metastases, none of the 13 patients receiving savo + osimertinib developed new CNS lesions at progression. In contrast, 6 of 11 patients (55%) in the placebo group did develop new CNS metastases at progression.
These findings suggest promising CNS protective activity of the combination therapy.
What This Means for Patients
The SAVANNAH randomized cohort provides robust clinical evidence supporting the dual targeting of EGFR and MET in patients with EGFRm NSCLC who progress on first-line osimertinib and harbor MET IHC3+ and/or MET FISH amplification. The combination of savolitinib and osimertinib resulted in higher response rates, longer duration of benefit, improved PFS, and fewer CNS relapses than MET inhibition alone.
This is currently one of the largest randomized datasets available assessing an oral MET-TKI in this resistance setting, offering a critical foundation for ongoing Phase III efforts, including the SAFFRON trial, which will further explore the role of this combination in a larger patient population.
What People Are Saying About the SAVANNAH Trial?
Noemi Reguart, MD/PhD, thoracic oncologist, professor, and translational research lead in targeted therapies, shared on X.
” Phase 3 SACHI study savolitinib plus osi vs. SoC ChT in METamp/TKI-resistant EGFRm aNSCLC (China): mPFS ITT 8.2 mo vs 4.5 (HR 0.34). PFS benefit regardless of prior TKI-generation. ORR 58 vs 34%. Quite consistent with SAVANNAH’s phase 2 trial (ELCC2025) ORR 56%, mPFS 7.4 mo) “