Marios Georgakis, a Physician-scientist leading a lab at Universität München, shared a post on X:
“I’m often asked how human genetics can be used to validate drug targets.
In our new Nature Cardiovascular Research paper, we provide an end-to-end framework for IL-6 inhibition
- build a genetic proxy
- demonstrate potential for cardiovascular benefit
- assess expected & unexpected safety signals.
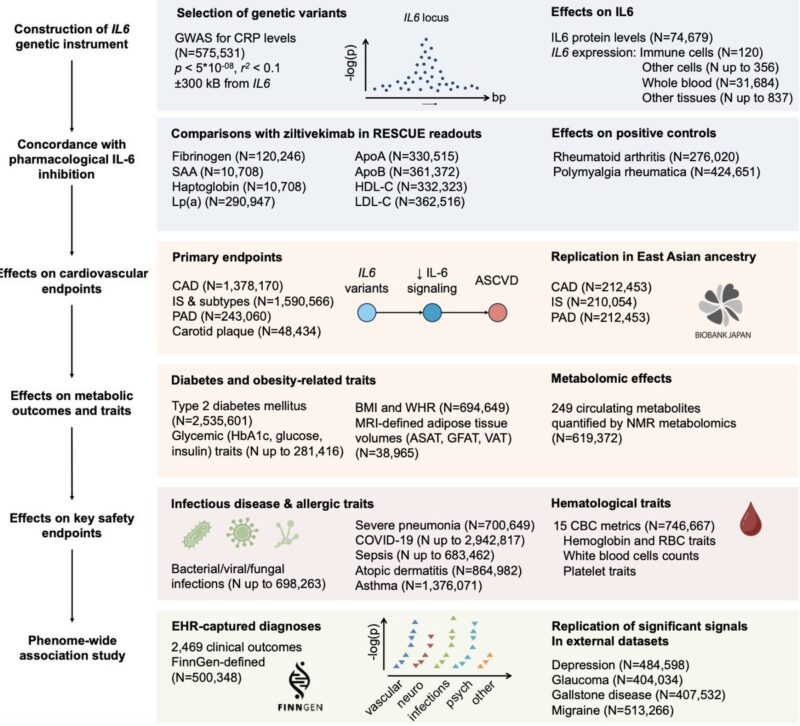
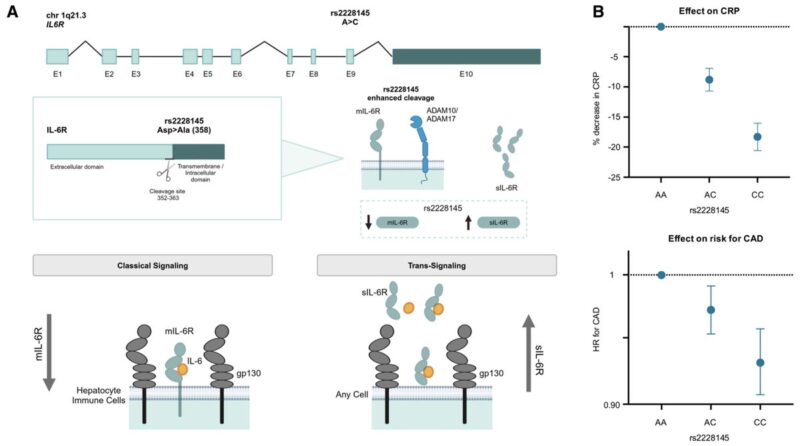
Genetic variation in IL6R, the receptor for IL-6, has long been linked to risk of atherosclerotic cardiovascular disease (ASCVD).
This has motivated the development of anti-inflammatory therapies targeting IL6 signaling for ASCVD.
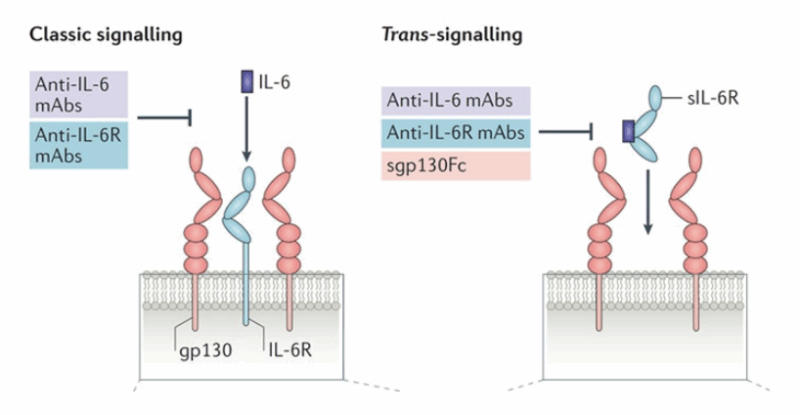
However, antibodies in development (ziltivekimab by Novo Nordisk, clazakizumab by CSL Behring, pacibekitug by Tourmaline Bio) target IL-6, not its receptor.
This raises a key translational question:
Can the genetic evidence for IL6R perturbation be applied to these therapeutics?
We screened the IL6 locus to identify variants associated with CRP, a downstream biomarker of IL-6 signaling and the primary endpoint in phase 2 trials of anti-IL-6 therapies.
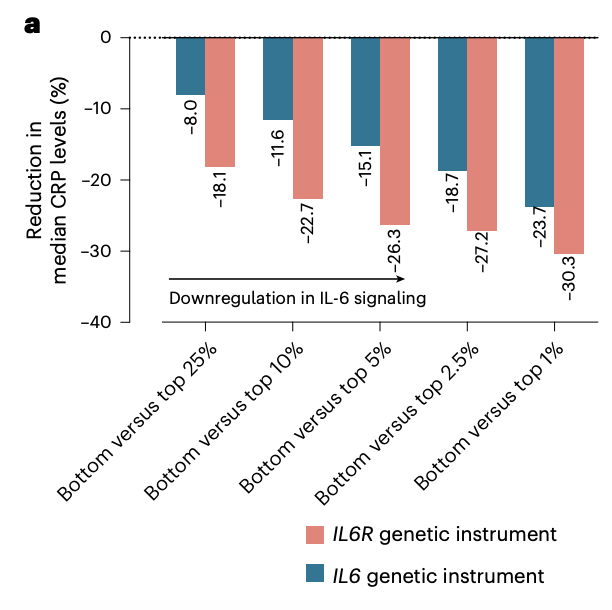
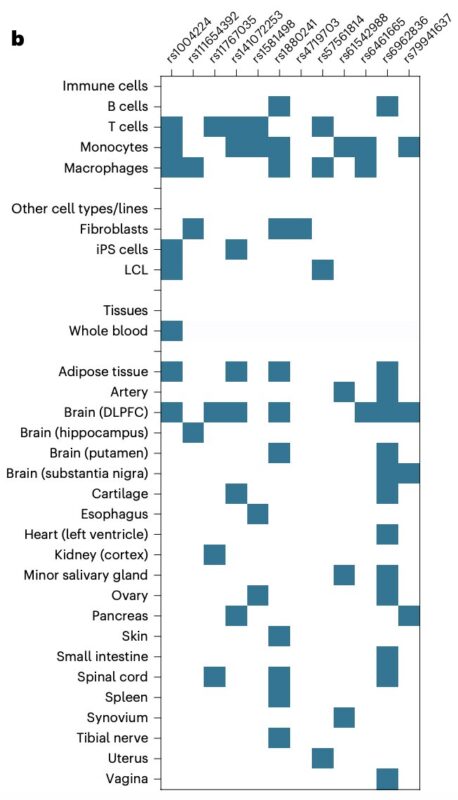
This identified 12 moderately correlated variants (r² < 0.1) linked to CRP levels.
Aggregating these 12 variants into a genetic score was associated with lifelong reductions in CRP (up to 24% for the upper vs lower percentile), comparable in magnitude to those observed with an IL6R genetic proxy.
All selected variants were non-coding, yet many were associated with IL6 expression in one or more cell types or tissues. This suggests they may modulate downstream signaling by altering IL6 RNA expression.
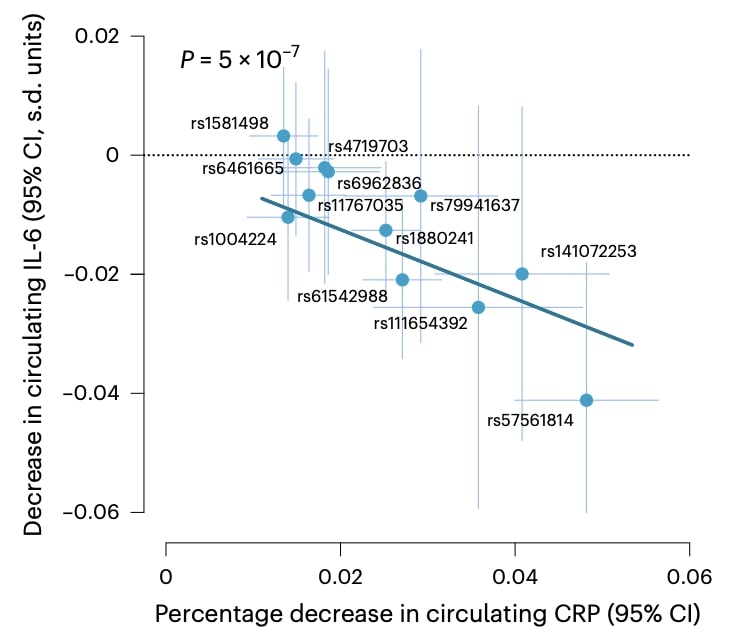
Indeed, most variants showed proportionally concordant effects on circulating IL-6 protein levels, supporting an effect on IL-6 abundance.
Can such a genetic proxy be used to study the effects of pharmacological IL-6 inhibition?
While phase 3 data are not yet available, phase 2 results from the RESCUE trial (ziltivekimab) provide biomarker evidence.
When indexed to CRP reduction, the effects of our genetic instrument on 8 tested biomarkers (data leveraged through publicly available GWASs) were highly concordant with ziltivekimab treatment!
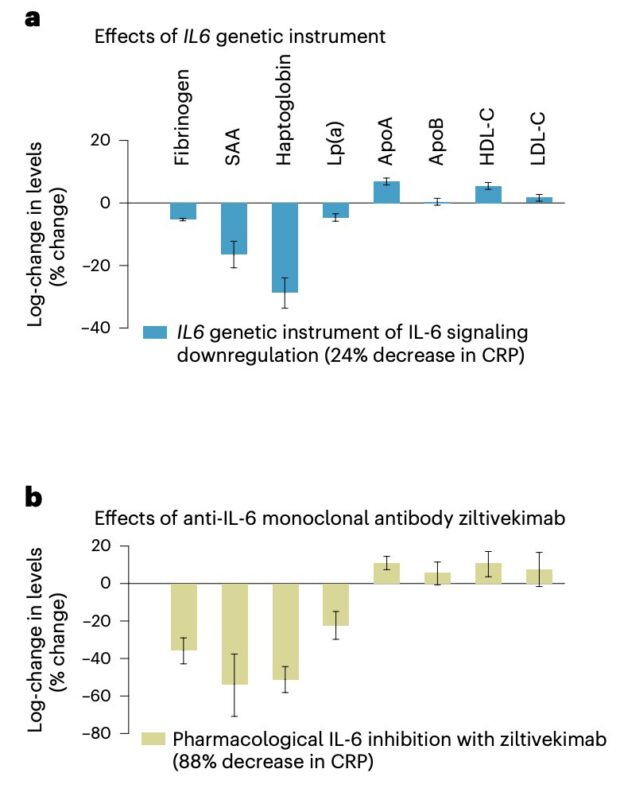
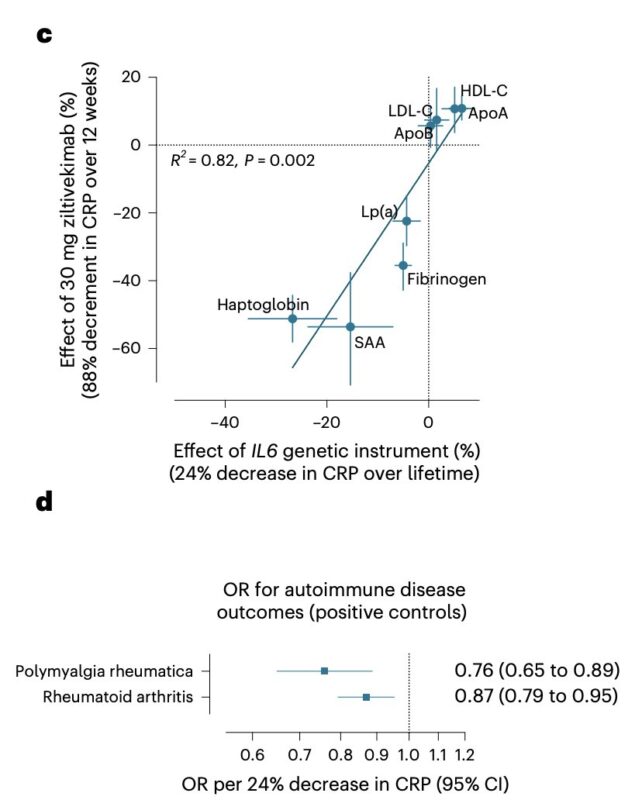
In other words, our genetic instrument can predict effects on all 8 biomarkers measured in the trial, with a correlation of 0.9 between genetic and trial effects.
Similarly, the IL-6 genetic proxy showed effects on rheumatoid arthritis and polymyalgia rheumatica, both indications where IL-6 inhibition has proven clinically effective.
We then tested associations with cardiovascular endpoints.
Like an IL6R instrument, genetically proxied IL-6 downregulation through IL6 perturbation was associated with lower risk of atherosclerotic outcomes:
- Coronary artery disease (CAD)
- Peripheral artery disease (PAD)
- Large artery atherosclerotic stroke
- Carotid atherosclerotic plaque
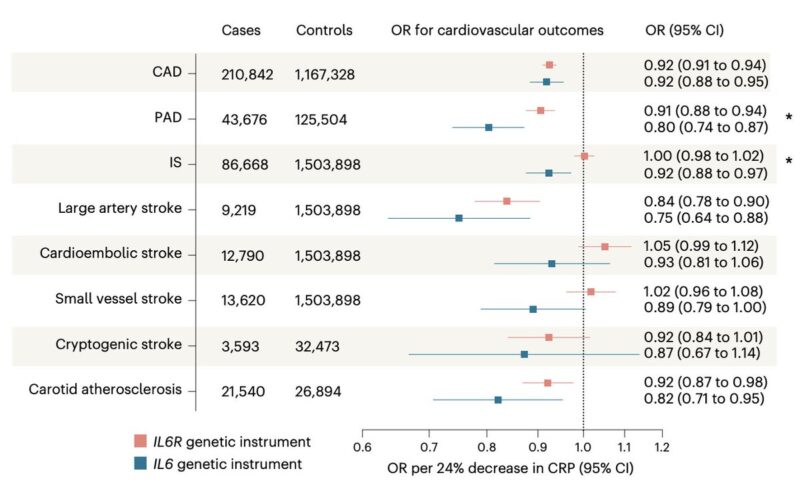
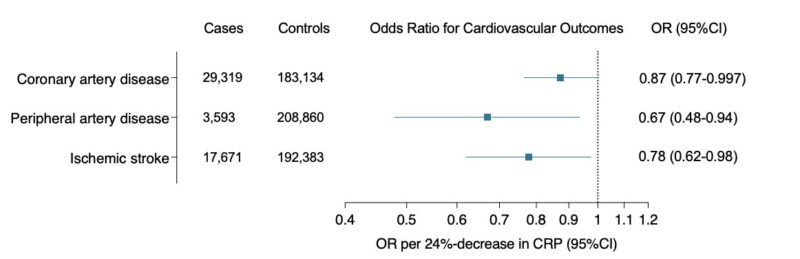
These findings were highly consistent in East Asian individuals from Biobank Japan.
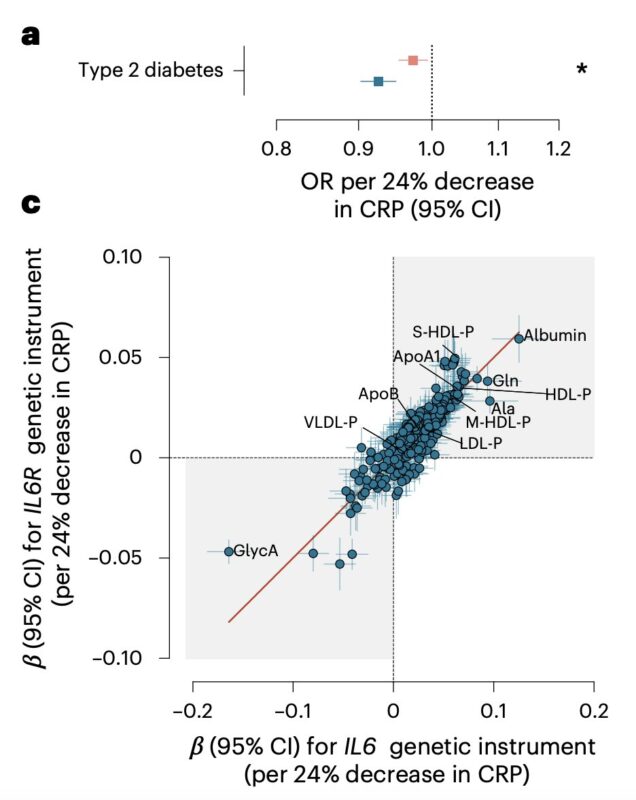
We also observed interesting metabolic effects:
- an association with a lower risk of type 2 diabetes
- significant changes in lipid profile, mainly increase in HDL particles
Across multiple metabolomic measurements, the effects were highly consistent with those of an IL6R instrument, indicating convergence downstream of IL-6 signaling
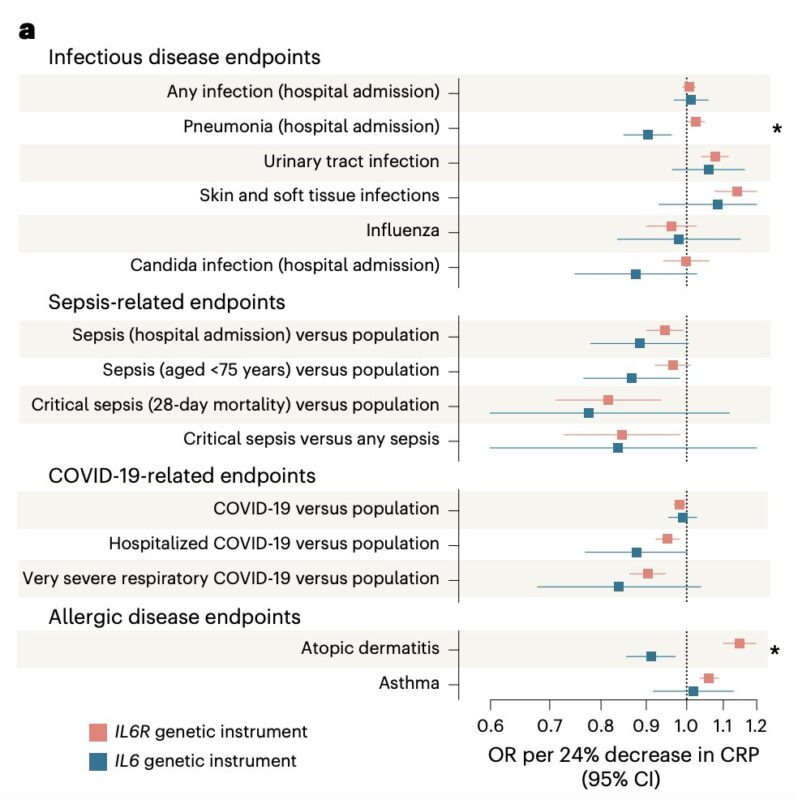
The primary safety concern for anti-IL-6 therapies is infection risk.
In previous work, we have shown that genetically proxied IL6R inhibition increases risk of bacterial infections, consistent with clinical experience with tocilizumab.
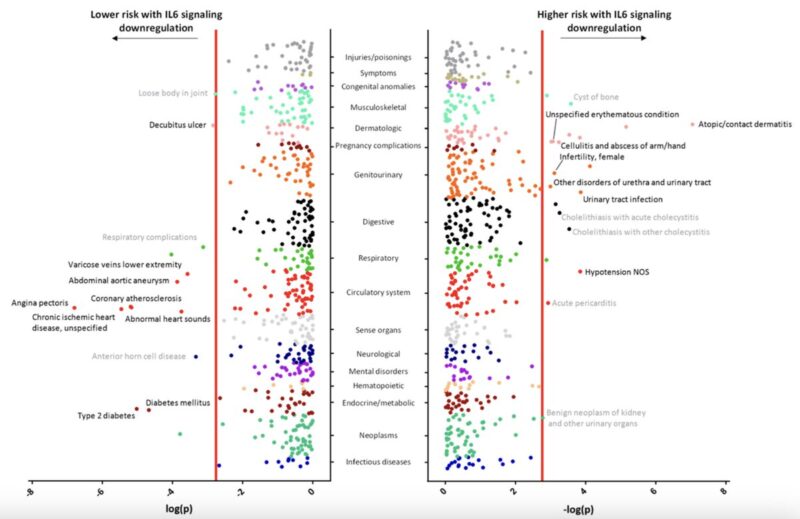
Interestingly, our IL-6 genetic proxy showed no significant increase in the infection endpoints tested.
On the contrary, there was even evidence of risk-lowering effects on pneumonia hospitalization.
Finally, we performed a phenome-wide association study (PheWAS) in FinnGen to systematically assess whether our IL-6 genetic proxy is associated with any additional outcomes beyond those already studied.
The main risk-lowering effects of the IL-6 proxy were replicated across:
- cardiovascular and metabolic endpoints
- autoimmune disease outcomes
- respiratory infections (pneumonia, influenza, COPD-related)
We also observed novel protective associations with:
- depression
- gallstone disease
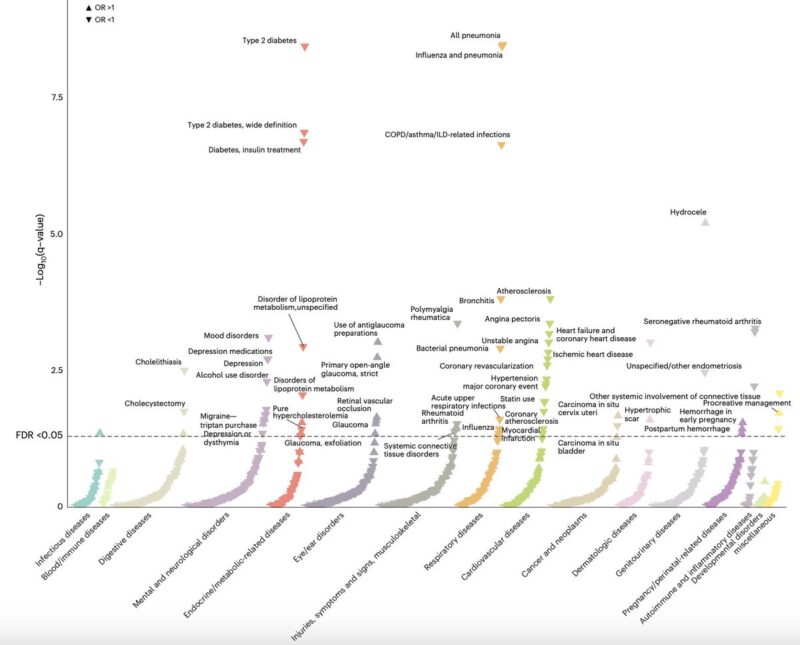
Regarding side effects, notable and consistent signals included:
- Glaucoma
- Perinatal and postpartum maternal hemorrhage
These may be relevant for specific populations and warrant further investigation.
Thoughts for broader implications:
1. Often, human genetic studies replicate trial findings retrospectively.
The results of ZEUS, a phase 3 cardiovascular outcomes trial testing ziltivekimab in patients with ASCVD and high CRP, are expected in 2026.
I hope this work will provide prospective validation for the use of human genetics to predict trial-relevant clinical outcomes.
2. Nearly all data used in this study were publicly available (except the individual-level CRP analysis in UK Biobank that requires submission of an application and payment for access).
In principle, this framework could be applied to genetically validate any drug target, particularly those developed for chronic, aging-related diseases.
3. Large-scale retrospective analyses of target-indication pairs (e.g., Minikel et al, Nature 2024) offer big picture evidence that genetically supported targets are more likely to lead to approved drugs.
However, each target and indication has unique nuances that necessitate individual tailored approaches for proper validation.
A scalable, one-size-fits-all approach risks overlooking important context and already acquired knowledge.
Great work by Lanyue Zhang, Murad Omarov, and Lingling Xu. Grateful for the partnership with TourmalineBio
(developing an anti-IL6 antibody) and the collaboration with Pradeep Natarajan.”
Title: IL6 genetic perturbation mimicking IL-6 inhibition is associated with lower cardiometabolic risk
Authors: Lanyue Zhang, Murad Omarov, Lingling Xu, Emil deGoma, Pradeep Natarajan, Marios K. Georgakis
Read the Full Article in Nature.

More posts featuring Marios Georgakis on OncoDaily.


