Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital and heads the Department of Haemostaseology, the Haemophilia Center of Medical Clinic, posted on LinkedIn:
“Six-Year Gene Therapy Data for Haemophilia B – presented by Dr. Ben Samelson-Jones at ISTH 2025. The remarkable long-term data from the phase 1/2a highlight the durability and safety of a single 5 × 10¹¹ vg/kg infusion of fidanacogene elaparvovec in 14 adults with haemophilia B (mean age 40.1 y; 14.3% HIV+)
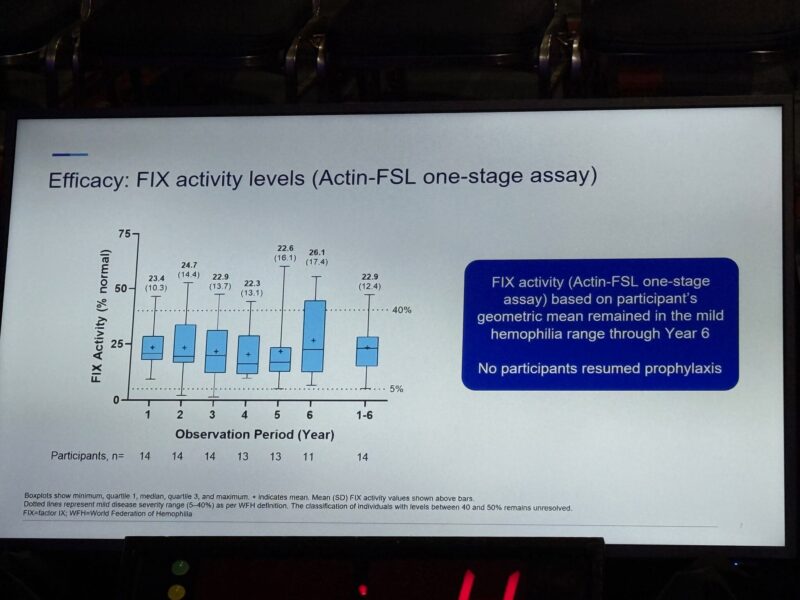
Efficacy at 6 years
· FIX activity held steady in the mild range (mean 22.3 – 26.1%)
· 13/14 participants (93%) stayed in the mild range throughout follow-up
· Zero bleeds in the majority (71 %) of participants
· No one resumed prophylaxis during the entire 6-year period
· Annualized bleeding rate remained < 1 from year 1 through year 6
Safety profile
· No treatment-related adverse events after year 1
· 5/14 had liver ultrasound findings deemed pre-existing or unrelated (steatosis, gallstones, cirrhosis progression)
· No thrombotic events, malignancies, or FIX inhibitors detected at any point
Commercial update
Despite these results, Pfizer withdrew Beqvez/Durveqtix worldwide in Feb 2025; the EU marketing authorization was formally withdrawn on 15 May 2025. Meanwhile, the BENEGENE-2 phase 3 trial is ongoing, further investigating the efficacy and safety of fidanacogene elaparvovec in 79 participants
These data remain a landmark proof-of-concept for durable, low-dose AAV gene therapy—offering key lessons as the field pivots to next-generation approaches.”
Read Further: 20 Posts Not To Miss From ISTH 2025.


