Gustavo Viani, Professor of Radiation Oncology at Ribeirão Preto Medical School, shared an article by Fabian Acker, et al. on X:
“Immunotherapy + cCRT in Stage III NSCLC: Breakthrough or Too Risky?
Objective:
- Assess efficacy & safety of immune checkpoint inhibitors (CPI) + concurrent chemoradiotherapy (cCRT).
Methods:
- 7 trials, 653 patients •Endpoints: Pneumonitis, ORR, PFS, OS.
Results:
- ORR: 69%.
- Median PFS: 16.3 months.
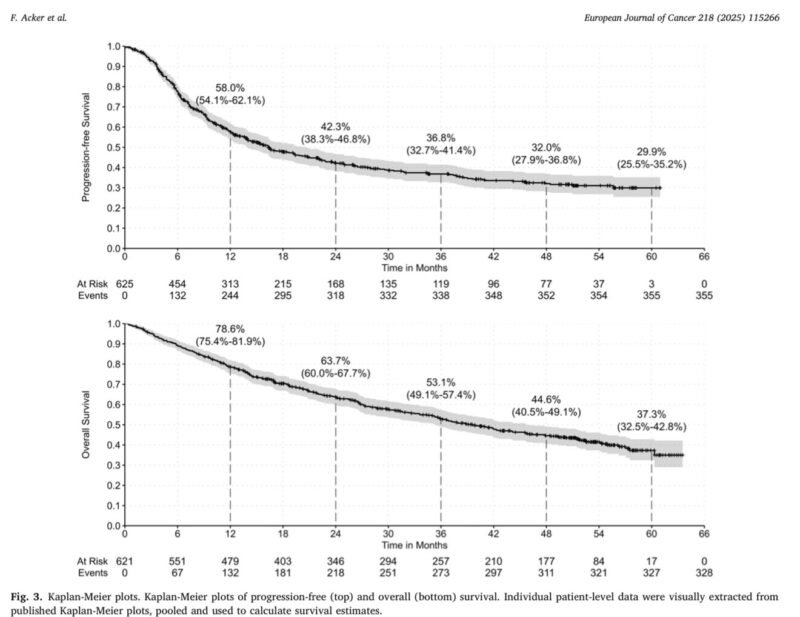
- Median OS: 39.5 months.
- 3-year OS: 53.1%.
Toxicity:
- Pneumonitis (any grade): 33%.
- Grade 3–5 pneumonitis: 7%.
- Dual CPI (PD-1 + CTLA4) → 16% mortality.
Takeaways:
- Single-agent CPI + cCRT is feasible.
- Dual CPI = high toxicity.
- More trials needed (CheckMate-73L, EA5181, APOLO).
Efficacy and safety of immune checkpoint inhibition combined with concurrent chemoradiotherapy in patients with stage III unresectable non-small cell lung cancer: A systematic review and meta-analysis.
Authors: Fabian Acker, et al.