At the ESMO Breast Cancer Congress 2025, Dr. Sherene Loi (Melbourne, Australia) presented findings from the Phase II DIAmOND trial (BCT1703), evaluating tremelimumab and durvalumab in combination with trastuzumab in patients with trastuzumab-resistant HER2-positive advanced breast cancer (ABC). While overall response rates were modest, signals of clinical activity emerged in ER-positive and PD-L1 positive subgroups, supporting further exploration of this dual checkpoint blockade strategy.
What Is the DIAmOND Trial?
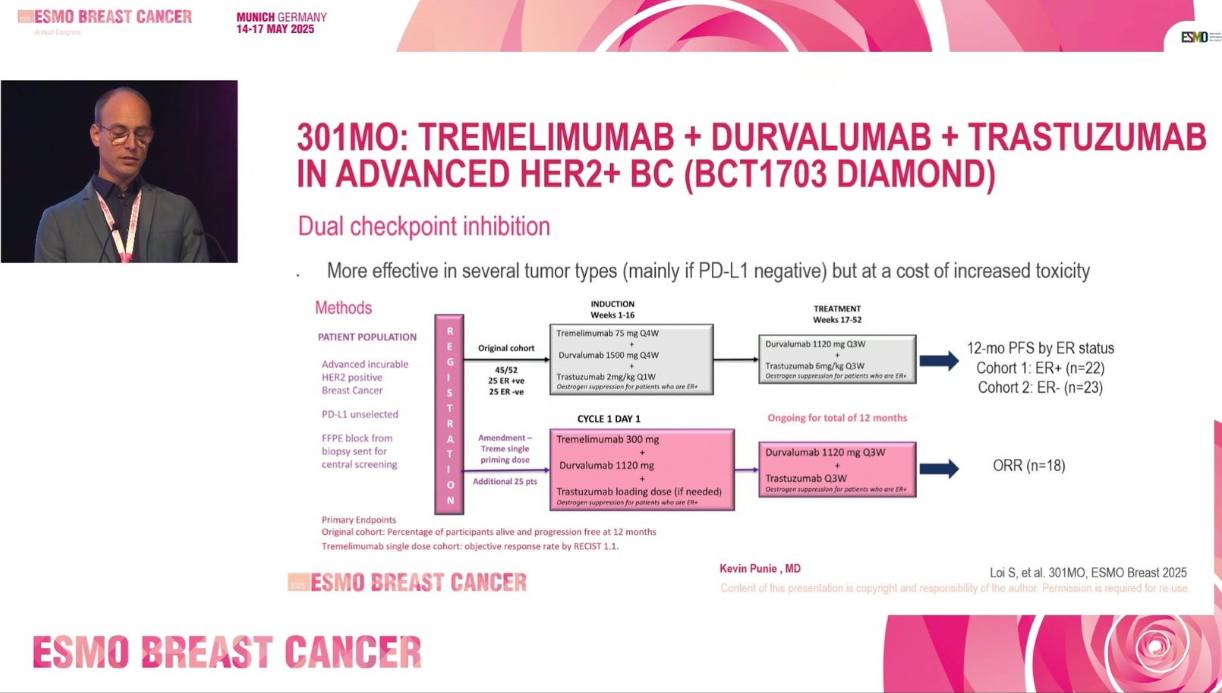
DIAmOND is a multicenter, single-arm Phase II study conducted across 11 Australian centers to assess the efficacy of dual immune checkpoint blockade—tremelimumab (CTLA-4 inhibitor) and durvalumab (PD-L1 inhibitor)—combined with trastuzumab in a heavily pretreated HER2-positive ABC population. The trial enrolled 72 patients who had received at least one prior trastuzumab-based regimen. Median prior lines of therapy were three. The study included three cohorts:
-
Cohort 1 (ER-positive) and Cohort 2 (ER-negative) received tremelimumab 75 mg Q4W with durvalumab 1500 mg Q4W and trastuzumab 2 mg/kg Q1W for 12 weeks, followed by durvalumab 1120 mg Q3W and trastuzumab 6 mg/kg Q3W.
-
Cohort 3 (ER-unselected) received a priming dose of tremelimumab 300 mg with the same durvalumab and trastuzumab maintenance.
ER-positive patients also received concurrent endocrine therapy.
Trial Endpoints and Translational Aims
The primary endpoint for Cohorts 1 and 2 was 12-month progression-free survival (PFS), while Cohort 3 focused on objective response rate (ORR) by RECIST. Secondary endpoints included ORR, clinical benefit rate (CBR), duration of response (DOR), safety, and iRECIST-based PFS. Exploratory analyses assessed tumor-infiltrating lymphocytes (TILs) and PD-L1 expression.
DIAmOND Trial Results
A total of 72 patients were enrolled across 11 sites, with a median age of 56 years (IQR 46–61). Among them, 68 were evaluable for the primary endpoint. The majority had received a median of three prior lines of therapy, reflecting a heavily pretreated cohort. Notably, only 5.9% had previously been exposed to trastuzumab deruxtecan (T-Dxd). Biomarker analyses revealed that 21% of patients were PD-L1 positive, and 41% had tumor-infiltrating lymphocytes (TILs) ≥5%.
Efficacy Outcomes
The DIAmOND trial demonstrated encouraging clinical activity in defined subgroups, particularly in ER-positive tumorsand patients receiving tremelimumab priming.
-
ER-positive patients achieved the highest objective response rate (ORR) at 27%, more than triple the rate seen in ER-negative tumors (8.7%). The tremelimumab-priming cohort also showed modest activity, with an ORR of 11%.
-
Progression-free survival (PFS) at 12 months was most favorable in the tremelimumab-priming arm (27%), compared to 16% in ER-positive and 8% in ER-negative cohorts.
-
When evaluated using iRECIST criteria, the overall 12-month PFS rose significantly to 53%, further supporting immunologic activity despite modest RECIST responses.
-
The clinical benefit rate (CBR)—defined as complete or partial response, or stable disease lasting ≥24 weeks—was 34% overall, with the highest benefit again in ER-positive patients (40%).
-
The mean duration of response (DOR) reached 19.9 months, with the most prolonged responses seen in ER-positive and tremelimumab-primed patients (Cohort 3).
These subgroup-driven findings point toward a potential role for dual checkpoint blockade, especially in biologically selected HER2+ populations.
Biomarker-Driven Insights
Among PD-L1 positive tumors, the overall ORR reached 38%, with a striking 100% response rate in ER-positive/PD-L1+ patients (2/2). Conversely, no responses were observed in PD-L1 positive ER-negative tumors. In patients with TILs ≥5%, the ORR was 25%, with the highest responses again occurring in ER-positive tumors (63%).
Safety Profile
The treatment regimen was associated with a notable incidence of toxicity. Grade ≥3 adverse events occurred in 66% of patients in Cohorts 1 and 2, and in 53% of patients in Cohort 3. Adverse events of special interest—primarily immune-related—were reported in over 65% of patients across all cohorts, consistent with the expected safety profile of dual immune checkpoint blockade.

Read Full Abstract on ESMO Official Website
What This Means for Patients
The DIAmOND trial provides early evidence that combining dual immune checkpoint inhibitors with trastuzumab may offer clinical benefit for select patients with trastuzumab-resistant HER2-positive advanced breast cancer—particularly those who are ER-positive or have PD-L1 positive tumors. While overall response rates were modest and treatment-related side effects were common, the findings highlight the importance of biomarker testing and support ongoing efforts to personalize immunotherapy combinations in HER2+ disease. These results may help shape future clinical trials and offer hope for patients with limited treatment options.
You Can Also Read ROSALINE Trial Reports 49% Response in Lobular Breast Cancer at ESMO 2025 by Oncodaily

Written by Toma Oganezova, MD


