Dr. Soraia Lobo Martins (Brussels, Belgium) presented results from the Phase II ROSALINE trial at the ESMO Breast Cancer Congress 2025, the first neoadjuvant endocrine therapy study dedicated exclusively to invasive lobular breast cancer (ILBC). Although the trial did not meet its primary endpoint of achieving RCB 0/1, it demonstrated a 49% objective response rate (ORR) by MRI and confirmed the feasibility of conducting subtype-specific trials in ILBC. Ongoing translational analyses aim to identify biomarkers predictive of response to ROS1 inhibition.
What Is the ROSALINE Trial?
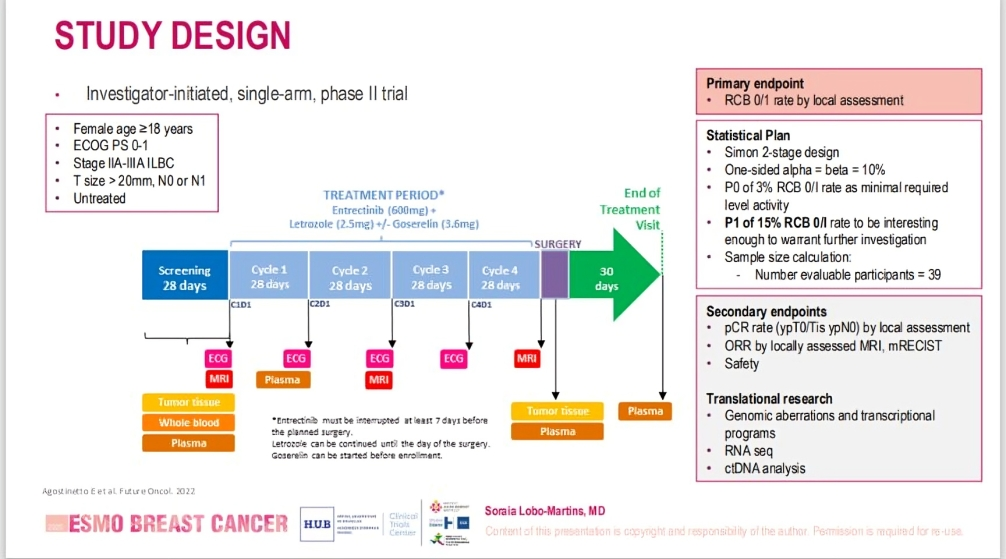
ROSALINE is the first neoadjuvant endocrine therapy (ET) trial dedicated exclusively to invasive lobular breast cancer (ILBC)—a subtype accounting for approximately 15% of all breast cancers, yet often treated similarly to ductal carcinoma despite its unique biology. ILBC is characterized by loss of E-cadherin (CDH1), which in preclinical studies has shown synthetic lethality with ROS1 inhibitors.
This investigator-initiated, single-arm, Phase II trial evaluated the activity of entrectinib—a ROS1 inhibitor—in combination with letrozole (2.5 mg/day) and goserelin (for premenopausal patients) in women with HR+/HER2– stage IIA–IIIA Invasive Lobular Breast Cancer. Patients received 4 cycles of therapy before undergoing surgery.
Trial Design and Objectives
The primary endpoint was the Residual Cancer Burden (RCB) 0/1 rate, a stringent marker of neoadjuvant treatment efficacy. Secondary endpoints included:
- Pathological complete response (pCR)
- Objective response rate (ORR) by MRI
Read Full Abstract on ESMO Official Website
Safety and tolerability
Patients were considered evaluable if they completed ≥80% of the planned entrectinib treatment. Additional translational endpoints—such as tumor-infiltrating lymphocytes (TILs), ctDNA dynamics, RNA sequencing, and central MRI review—are ongoing.
ROSALINE Trial Results
Out of 56 patients enrolled, 41 were evaluable for the primary endpoint:
- RCB 0/1 rate: 0% (primary endpoint not met)
- MRI-based ORR: 49% (CR 10%, PR 39%)
- pCR: 0% (one patient not evaluable for RCB achieved pCR)
- Most common adverse events: constipation (71%), dysgeusia (61%)
- Grade ≥3 AEs: 22%
- Dose reductions: occurred in 61% of patients
- Treatment discontinuation: 59% of evaluable patients
Despite the absence of a major pathological response, radiologic activity was evident, and biomarker analyses are ongoing to better understand which subgroups may benefit from ROS1 inhibition.
Key Takeaways
First neoadjuvant ET trial focused on ILBC—a significant achievement for this under-studied subtype
- RCB 0/1 and pCR rates were 0%, but MRI-based ORR was nearly 50%
- High rate of dose reductions and discontinuations suggests tolerability challenges with entrectinib
- Translational research is ongoing, which may help identify predictive biomarkers for ROS1-targeted therapy
The trial highlights the importance and feasibility of lobular-specific clinical trials
What People Are Saying About the ROSALINE Trial
Dr. Guilherme Nader Marta from Dana-Farber Cancer Institute, shared on X
“ROSALINE, led by Dr. Martine Piccart and presented by Dr. Soraia Lobo Martins, is the first preoperative endocrine therapy trial dedicated to lobular breast cancer. Although the primary endpoint was not met, ORR by MRI was 49%. Biomarker analyses are underway. Another example that ILC-focused trials are feasible!”
Dr. Jason A. Mouabbi, MD Anderson Cancer Center, shared on X
“The #ROSALINE trial results are here! Did not meet primary endpoint but #ROS1i #Entrectinib has shown activity in early stage #ILC. Our study #REPLOT using the next-gen #ROS1i #Repotrectinib is currently enrolling #mILC in the US.”
“Mini oral session at ESMOBreast25 with @S_LoboMartins from JulesBordet presenting results of ROSALINE trial showing limited activity of neoadjuvant entrectinib plus endocrine therapy in lobular BreastCancer”

While the ROSALINE trial did not meet its primary endpoint of achieving RCB 0/1, it remains a pivotal effort in reshaping how ILBC is studied and treated. The encouraging MRI-based responses and commitment to molecular profiling suggest that targeted strategies for lobular breast cancer are both possible and necessary. The oncology community now looks ahead to emerging studies—like REPLOT—that may build on these insights and deliver more tailored options for this distinct breast cancer subtype.
More updates on ESMO Breast 2025 on OncoDaily


