Chris De Savi, Curie.Bio posted on LinkedIn:
“New Winning Drugs in ER+ Breast Cancer?
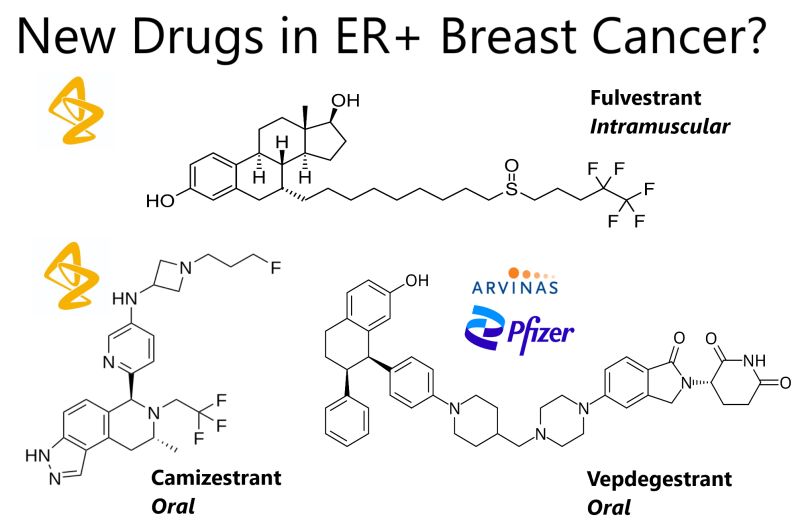
The treatment landscape for advanced estrogen receptor (ER)–positive, HER2-negative breast cancer is evolving with novel oral selective estrogen receptor degraders (SERDs). Two such agents, vepdegestrant and camizestrant, have been evaluated in clinical trials, offering insights into their potential to replace the standard intramuscular SERD, fulvestrant. Hot off the press hashtag#ASCO25!
In the VERITAC-2 Phase 3 trial, published in NEJM yesterday, vepdegestrant was compared to fulvestrant in patients who had progressed on prior endocrine therapy and a CDK4/6 inhibitor. In the overall population, vepdegestrant did not significantly improve PFS (3.8 vs. 3.6 months; HR, 0.86; 95% CI, 0.70–1.06; P=0.16).
However, in patients with ESR1 mutations, vepdegestrant extended median PFS to 5.0 months versus 2.0 months for fulvestrant (HR, 0.60; 95% CI, 0.43–0.83; P=0.002).
Meanwhile, camizestrant has shown broader efficacy in the SERENA-6 trial published in NEJM today. This Phase 3 study involved patients on first-line aromatase inhibitor plus CDK4/6 inhibitor therapy. ESR1 mutations were tracked via ctDNA, and patients with these mutations were randomized to switch to camizestrant plus the same CDK4/6 inhibitor or continue standard therapy.
Median PFS was 16.0 months for camizestrant versus 9.2 months for continued standard therapy, a 56% reduction in risk (HR, 0.44; 95% CI, 0.32–0.60; P<0.001). Camizestrant plus CDK4/6 inhibitors was well-tolerated with low discontinuation rates.
Compared to fulvestrant, which is limited by its intramuscular administration and median PFS of 3.6 months, vepdegestrant (oral) offers targeted benefit in ESR1-mutant disease with PFS of 5.0 months. Camizestrant, also oral, demonstrated broader efficacy with a median PFS of 16.0 months in patients with ESR1 mutations detected through liquid biopsy while on first-line therapy.
Both oral SERDs represent a major advance, offering convenient administration and potential to overcome resistance mechanisms. Vepdegestrant’s activity in ESR1-mutant disease highlights its targeted promise, while camizestrant’s robust efficacy and proactive treatment strategy may establish it as a new standard of care.
These findings suggest a dynamic shift in the endocrine therapy landscape, with new options poised to replace fulvestrant and improve outcomes for patients with advanced ER-positive, HER2-negative breast cancer. Really very exciting for patients!”
More posts featuring ASCO25.