Yakup Ergün, Medical Oncologist at Antalya City Hospital, shared a recent article by Thierry Andre et al. on X:
“The New Standard of Care in MSI-H CRC: Nivolumab + Ipilimumab.
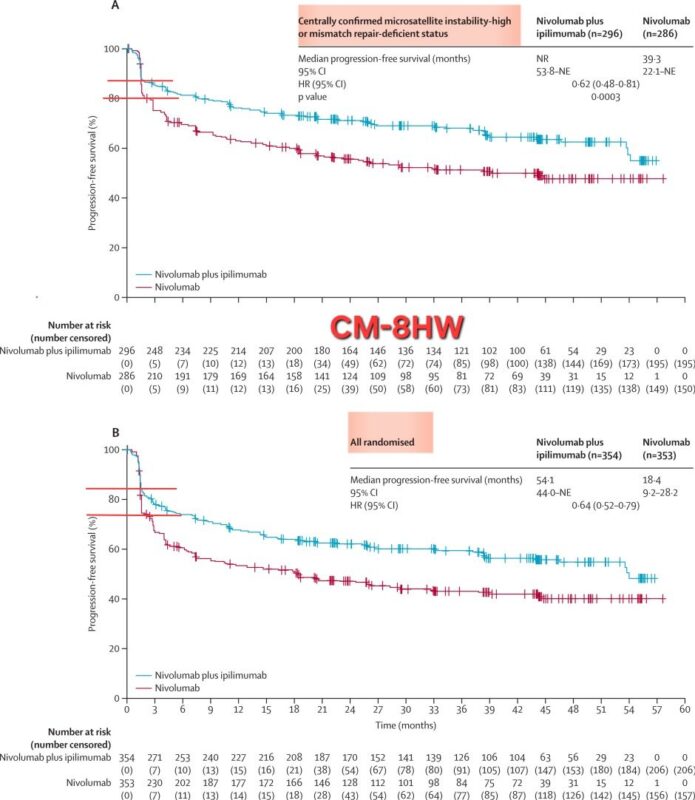
The CM-8HW trial has provided us with some important insights:
1. Discrepancy between local and central MSI testing:
- In the CM-8HW study, 14% of patients who were initially classified as MSI-H based on local testing were found to be non-MSI-H upon central assessment. This discrepancy is significant, as in the central analysis, PFS in the nivolumab + ipilimumab arm did not reach NR, compared to 39.3 months in the nivolumab arm. However, when analyzed based on local lab results, PFS outcomes were notably lower (54.1 months vs. 18.4 months).
- This data is not available in the KEYNOTE-177 study. Considering that KEYNOTE-177 started earlier and pathologists likely had less experience with MSI testing at that time, the rate of incorrect MSI-H classification could be higher.
2. Early progression:
- Although MSI-H tumors are generally sensitive to ICIs, a subset of patients experiences early progression. One key reason could be inaccurate MSI-H testing. In the CM-8HW study, survival curves based on central and local MSI-H validation showed that early progression rates (within the first three months) were lower in the central assessment.
- In KEYNOTE-177, the early progression rate was as high as 35% in the first three months. In CM-8HW, when centrally confirmed MSI-H patients were analyzed, the early progression rate in the nivolumab + ipilimumab arm was around 15%, and approximately 20% in the nivolumab monotherapy arm (compared to 25% based on local testing).
- This emphasizes the importance of accurate MSI-H testing and suggests that dual ICI inhibition may be more effective in overcoming primary resistance compared to single-agent ICI therapy. (A question remains: how would an ICI + chemotherapy combination perform in this scenario?)
In summary, while ICIs have revolutionized the treatment of MSI-H tumors, ensuring accurate MSI testing and addressing primary resistance through effective combinations can further improve outcomes.”
Authors: Thierry Andre, Elena Elez, Eric Van Cutsem, Lars Henrik Jensen, Jaafar Bennouna, Guillermo Mendez, Michael Schenker, Christelle de la Fouchardiere, Maria Luisa Limon, Takayuki Yoshino, Jin Li, Heinz-Josef Lenz, Jose Luis Manzano Mozo, Giampaolo Tortora, Rocio Garcia-Carbonero, Laetitia Dahan, Myriam Chalabi, Rohit Joshi, Eray Goekkurt, Maria Ignez Braghiroli, Timucin Cil, Elvis Cela, Tian Chen, Ming Lei, Matthew Dixon, Sandzhar Abdullaev and Sara Lonardi

More posts featuring Yakup Ergün.


