Toni Choueiri, Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute, posted on X about recent paper by him as first author, titled “Avelumab + axitinib vs sunitinib as first-line treatment for patients with advanced renal cell carcinoma: final analysis of the phase 3 JAVELIN Renal 101 trial” published on ESMO Annals of Oncology.
Authors: T.K. Choueiri, K. Penkov, H. Uemura, M.T. Campbell, S. Pal, C. Kollmannsberger, J.L. Lee, B. Venugopal, A.J.M. van den Eertwegh, S. Negrier, H. Gurney, L. Albiges, R. Berger, J.B.A.G. Haanen, V. Oyervides Juárez, B.I. Rini, J. Larkin, F. Nolè, M. Schmidinger, M.B. Atkins, Y. Tomita, B. Ellers-Lenz, J. Hoffman, R. Sandner, J. Wang, A. di Pietro, R.J. Motzer

“Excited to share the FINAL analysis of the JAVELIN Renal 101 trial, now published in ESMO Annals of Oncology!
JAVELIN Renal 101 is a randomized phase 3 multicenter trial. We enrolled patients with:
- untreated advanced RCC with a clear-cell component.
- ECOG of 0 or 1 TX.
- Avelumab (PDL1 inh) + axitinib (VEGF TKI).
- Sunitinib monotherapy.
Of 886 randomized patients, 442 received avelumab + axitinib and 444 sunitinib, with 270 and 290 having PD-L1+ tumors respectively.
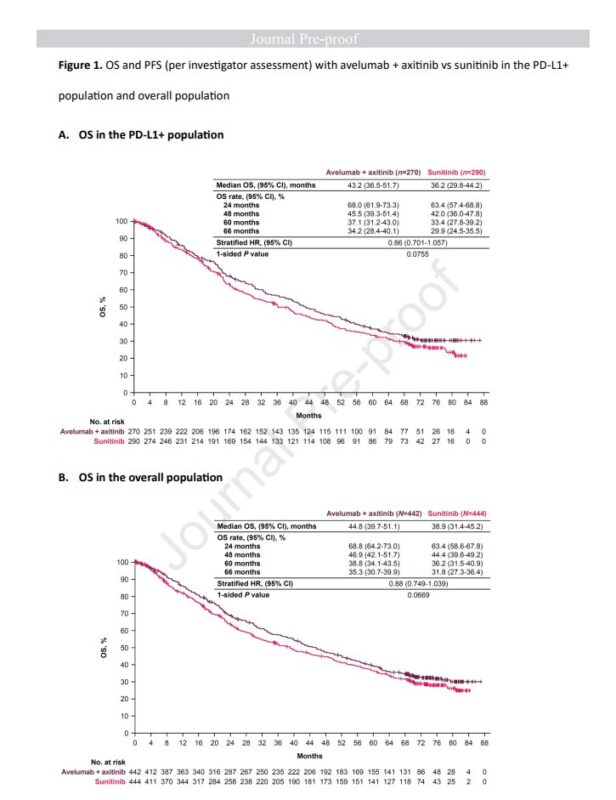
Median OS for avelumab + axitinib was 44.8 months, vs. 38.9 months for sunitinib (HR= 0.88, 95% CI= 0.749-1.039).
In PD-L1+ patients, Median OS was 43.2 months with avelumab + axitinib vs. 36.2 months for sunitinib (HR=0.86).
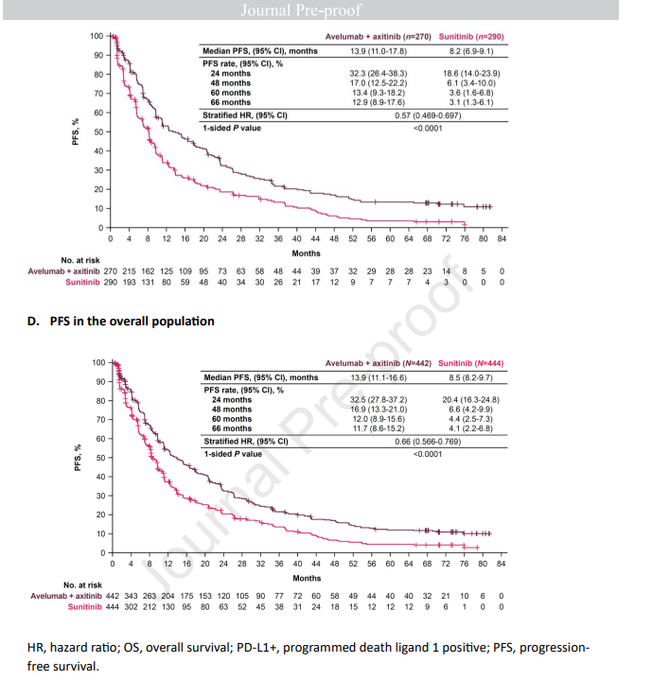
Delving further into PFS: 12% of patients achieved a 5-year PFS with avelumab + axitinib vs. 4.4% with sunitinib (95% CI, 0.566-0.769). In PD-L1+ patients: 5-year PFS rate of 13.4% vs. 3.6% (95% CI, 0.469-0.697).
Responses were more favorable in the combo group, with a nearly doubled ORR of 59.7% vs. 32% in the sunitinib group. Depth/duration of response were also significantly improved.
This trial marks the longest follow-up yet for any ICI + TKI combo in advanced RCC. Though OS benefits didn’t reach statistical significance, avelumab + axitinib showed notable PFS improvements, doubled ORR, and durable responses with a manageable safety profile. While avelumab + axitinib shows efficacy, it’s accompanied by a higher incidence of grade ≥3 adverse events (66.8%) compared to sunitinib (61.5%). Despite this, patient-reported outcomes reveal minimal differences in QL between the two treatments.
Deep appreciation to patients, their families, researchers, and healthcare professionals. Your dedication and commitment are the driving forces behind these advancements in RCC treatment. Thank you all for your invaluable contributions!”
Toni Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. He is the Medical Director of International Strategic Initiatives at Dana-Farber Toni Choueiriand past President of the Medical Staff at DFCI (2016-2018).
He received the George Canellos Award for Excellence in Clinical Investigation and Patient Care from DFCI in 2013, the Eugene Schonfeld Award from the Kidney Cancer Association (KCA) in 2016, and is a 2021 Giants of Cancer Care inductee. He serves on the National Comprehensive Cancer Network (NCCN) Kidney Cancer Panel, KidneyCan Board, the National Cancer Institute (NCI) GU Steering Committee, and is past Chairman (2015-2018) of the Medical and Scientific Steering Committee of the KCA.
Dr. Choueiri is an elected member of the American Society of Clinical Investigation (ASCI). In addition, he is an Aresty Scholar from the Wharton School of Business at the University of Pennsylvania.