TROPION-Breast01 evaluated the TROP2-directed antibody–drug conjugate datopotamab deruxtecan (Dato-DXd) versus investigator’s choice of single-agent chemotherapy in patients with hormone receptor–positive, HER2-negative (HR+/HER2−) metastatic breast cancer who had progressed after endocrine therapy and prior chemotherapy.
In this heavily pretreated population, the study previously demonstrated a statistically significant and clinically meaningful improvement in progression-free survival, reflecting the evolving role of ADCs in a disease setting where historical chemotherapy options have offered limited durability and substantial toxicity.
Title: Datopotamab deruxtecan versus chemotherapy in previously treated inoperable/metastatic hormone-receptor-positive HER2-negative breast cancer: final overall survival analysis of the phase 3 TROPION-Breast01 study
Authors: B. Pistilli, K. Jhaveri, S.-A. Im, S. Pernas, M. De Laurentiis, S. Wang, N.Martínez Jañez, G. Borges, D.W. Cescon, M. Hattori, Y.-S. Lu, E. Hamilton, J. Tsurutani, K. Kalinsky, P.E.Rubini Liedke, D. Carroll, S. Khan, H.S. Rugo, B. Xu, A. Bardia
Published in Annals of Oncology, December 2025
Background
Hormone receptor–positive, HER2-negative (HR+/HER2−) metastatic breast cancer represents the most common molecular subtype of advanced breast cancer. While endocrine therapy combined with CDK4/6 inhibitors remains the standard first-line approach, disease progression is inevitable for most patients. Once endocrine resistance develops and further endocrine therapy is no longer suitable, treatment options historically relied on single-agent chemotherapy, which is associated with modest efficacy and cumulative toxicity, and often fails to provide a meaningful overall survival (OS) benefit. The introduction of antibody–drug conjugates (ADCs) has expanded the therapeutic landscape in later lines, particularly with agents targeting TROP2 and HER2.
Datopotamab deruxtecan (Dato-DXd), a TROP2-directed ADC, previously demonstrated a statistically significant and clinically meaningful improvement in progression-free survival (PFS) versus investigator’s choice chemotherapy in the phase III TROPION-Breast01 trial. The present analysis reports the final OS results, providing critical insight into the survival impact of Dato-DXd in an era increasingly shaped by subsequent lines of active therapy.
Methods
The final OS analysis was conducted according to the prespecified statistical plan of the TROPION-Breast01 study. Overall survival was defined as the time from randomization to death from any cause. Survival was compared between treatment arms using a stratified log-rank test, adjusting for key stratification factors applied at randomization.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using a stratified Cox proportional hazards model. Because multiple effective therapies became available during the conduct of the trial, including other ADCs, a post-hoc sensitivity analysis using inverse probability censoring weighting (IPCW) was performed to adjust for imbalances in subsequent ADC use between arms. Secondary efficacy endpoints, including investigator-assessed PFS, objective response rate (ORR), duration of response (DoR), disease control rate (DCR), PFS2, and time-to-event measures reflecting treatment sequencing, were updated at the final OS data cut-off. Safety was evaluated in all patients who received at least one dose of study treatment.
Study Design
TROPION-Breast01 was a global, randomized, open-label, phase III trial enrolling adults with inoperable or metastatic HR+/HER2− breast cancer. Eligible patients had experienced disease progression on endocrine therapy and were considered unsuitable for further endocrine treatment, and had received one or two prior lines of chemotherapy in the metastatic setting.
HER2 negativity was defined according to ASCO/CAP guidelines and included tumors with IHC 0, 1+, or 2+ with negative in situ hybridization.A total of 732 patients were randomized 1:1 to receive either
- Dato-DXd (6 mg/kg intravenously every 3 weeks) or
- investigator’s choice of chemotherapy, which included eribulin, capecitabine, vinorelbine, or gemcitabine.
Randomization was stratified by number of prior chemotherapy lines (1 vs 2), geographic region, and prior use of a CDK4/6 inhibitor. The trial had dual primary endpoints of PFS by blinded independent central review and OS, and was considered positive if either endpoint reached statistical significance.
Results
At the final OS data cut-off, the median follow-up was 22.8 months. Overall survival events occurred in 61.1% of patients in the Dato-DXd arm and 58.0% in the chemotherapy arm. The final OS analysis showed no statistically significant difference between treatment groups, with an HR of 1.01 (95% CI 0.83–1.22; P = 0.9445). Median OS was 18.6 months with Dato-DXd compared with 18.3 months with investigator’s choice chemotherapy. OS rates were similar across time points, with 12-month survival of 74.2% versus 71.1%, and 18-month survival of 52.0% versus 50.9%, respectively.
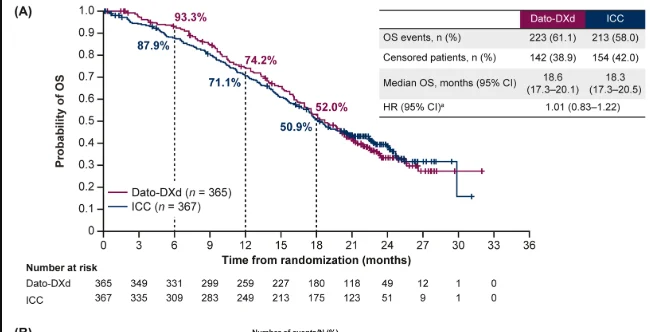
A key finding influencing OS interpretation was the imbalance in subsequent ADC use. After discontinuation of study treatment, 24.0% of patients in the chemotherapy arm received a subsequent ADC, compared with 12.3% in the Dato-DXd arm. When OS was adjusted for subsequent ADC therapy using the IPCW method, a numerical improvement favoring Dato-DXd emerged, with an adjusted HR of 0.86 (95% CI 0.70–1.06) and adjusted median OS of 19.1 months versus 17.5 months.
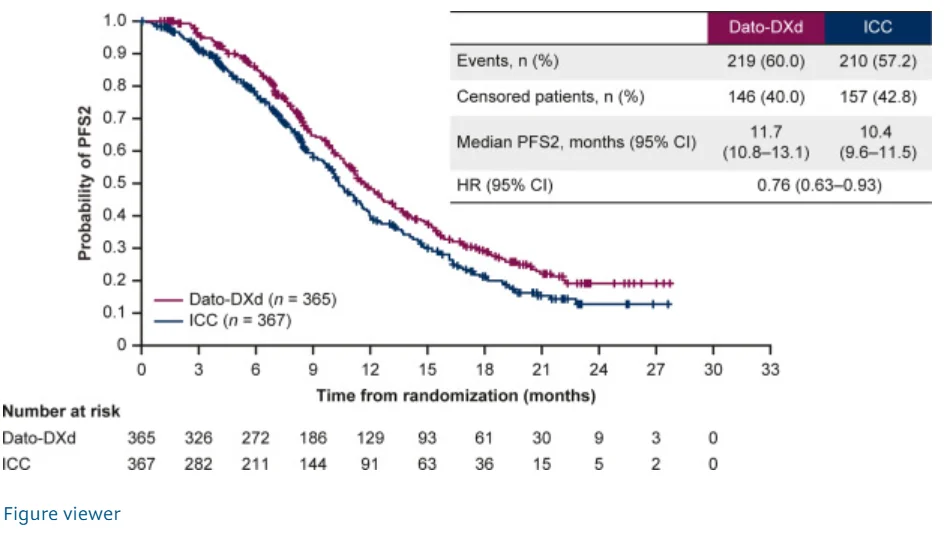
Secondary efficacy endpoints consistently favored Dato-DXd. Investigator-assessed PFS remained improved, with an HR of 0.64 (95% CI 0.55–0.76). The confirmed ORR was 36.7% with Dato-DXd compared with 22.1% with chemotherapy, and median DoR was 7.2 months versus 6.0 months, respectively. Disease control at 12 weeks was achieved in 80.8% of patients receiving Dato-DXd versus 66.8% with chemotherapy. Measures reflecting treatment sequencing also favored Dato-DXd, including PFS2 (11.7 vs 10.4 months; HR 0.76), time to first subsequent therapy (8.0 vs 5.2 months; HR 0.58), and time to second subsequent therapy (13.7 vs 12.3 months; HR 0.83).
Key Findings
The trial confirmed that Dato-DXd significantly prolongs PFS compared with standard chemotherapy in previously treated HR+/HER2− metastatic breast cancer. Although the final OS analysis was statistically neutral, extensive use of effective subsequent ADCs in the chemotherapy arm likely attenuated survival differences. Importantly, multiple secondary endpoints demonstrated consistent benefit with Dato-DXd, supporting durable disease control beyond first progression.
Key Takeaway Messages
Dato-DXd provides superior disease control compared with single-agent chemotherapy, with higher response rates, longer PFS, and improved downstream treatment metrics. OS interpretation in modern metastatic breast cancer trials is increasingly confounded by post-progression therapies, particularly ADCs. Secondary endpoints and treatment sequencing outcomes are therefore critical to understanding true clinical benefit. The favorable safety profile of Dato-DXd, with substantially lower rates of grade ≥3 treatment-related adverse events than chemotherapy, further supports its role in this setting.
Conclusion
The final OS analysis of TROPION-Breast01 confirms that datopotamab deruxtecan delivers meaningful clinical benefit through improved progression-free survival, higher response rates, and sustained disease control in patients with previously treated HR+/HER2− metastatic breast cancer. While overall survival was not statistically prolonged, imbalances in subsequent ADC therapy likely influenced this outcome. In the context of an evolving therapeutic landscape, the totality of efficacy and safety data supports Dato-DXd as a valuable treatment option and reinforces the need for refined approaches to survival assessment and ADC sequencing in advanced breast cancer.
You can read the full article here.


