At ASCO GI 2026, Stephen L. Chan presented the results of the phase III KEYNOTE-937 trial evaluating adjuvant pembrolizumab versus placebo in patients with hepatocellular carcinoma (HCC) who achieved a complete radiologic response following surgical resection or local ablation.
Although surgery and ablation can be curative in early-stage HCC, recurrence rates remain high, and no adjuvant systemic therapy has demonstrated a clear survival benefit. KEYNOTE-937 was designed to determine whether PD-1 inhibition could reduce recurrence risk and improve long-term outcomes in this setting.
Title: Adjuvant Pembrolizumab for Participants With Hepatocellular Carcinoma and Complete Radiologic Response After Surgical Resection or Local Ablation: The Phase III KEYNOTE-937 Study
Authors: Stephen L. Chan, Mohamed Bouattour, Thomas Yau, Ann-Lii Cheng, Yabing Guo, Chuang Peng, Do Young Kim, Lipika Goyal, Long-Bin Jeng, Ming-Chin Yu, Seung Woon Paik, Valeriy V. Breder, Robert C. Martin II, Arndt Vogel, Masatoshi Kudo, Jimin Wu, Usha Malhotra, Abby B. Siegel, Josep Llovet, Jia Fan.
Study Design and Methods
KEYNOTE-937 (NCT03867084) was a global, randomized, double-blind, placebo-controlled phase III study. Eligible patients were adults with confirmed HCC, ECOG performance status 0–1, Child-Pugh class A liver function, and a complete radiologic response after curative-intent surgical resection or local ablation. Patients with prior or ongoing hepatitis C infection or controlled hepatitis B infection were eligible if prespecified criteria were met.
Participants were randomized in a 1:1 ratio to receive pembrolizumab at a dose of 200 mg intravenously every three weeks or matching placebo, for up to 1 year or until disease recurrence, unacceptable toxicity, intercurrent illness, withdrawal, or study discontinuation. Randomization was stratified by geographic region, prior local therapy (resection versus ablation), recurrence risk, and alpha-fetoprotein level at diagnosis. The data cutoff for this third interim analysis (IA3) was March 20, 2025.
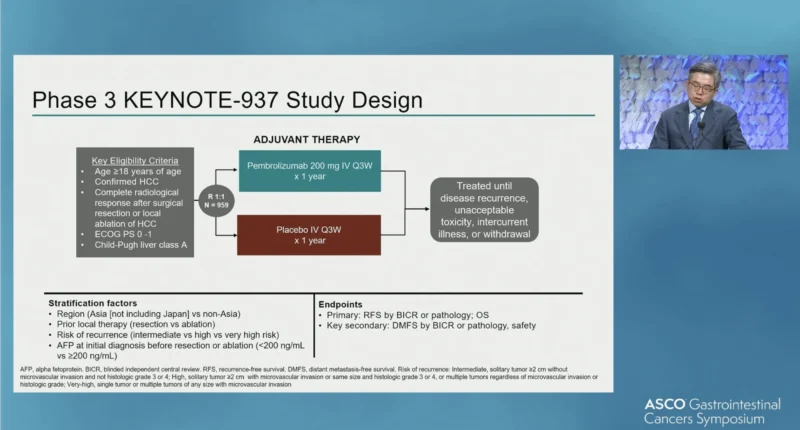
Endpoints
The primary endpoint was recurrence-free survival (RFS). Overall survival (OS) was hierarchically tested only if the RFS endpoint was met, per the prespecified multiplicity strategy. Key secondary endpoints included distant metastasis-free survival and safety.
Results
A total of 959 patients were randomized, with 476 assigned to pembrolizumab and 483 to placebo. At IA3, the median follow-up was 50.7 months (range, 31.6–69.1 months).
Key efficacy outcomes included:
- Recurrence-free survival: Median RFS was 46.7 months with pembrolizumab and 45.5 months with placebo (HR 1.06; 95% CI, 0.88–1.26; P = 0.719). The 48-month RFS rate was identical at 50% in both arms.
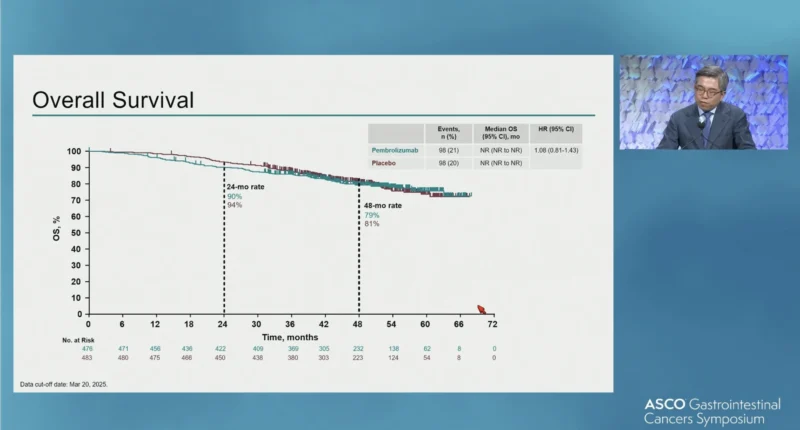
- Overall survival: Median OS was not reached in either group (HR 1.08; 95% CI, 0.81–1.43); OS was not statistically tested at IA3 because the RFS endpoint was not met, per the prespecified multiplicity strategy.
- Distant metastases-free survival (DMFS), a key secondary endpoint assessed by blinded independent central review or pathology, was not different between groups; median DMFS was not reached in either arm (HR 0.98; 95% CI, 0.77–1.24), and the 48-month DMFS rate was 71% with pembrolizumab and 70% with placebo.
Safety
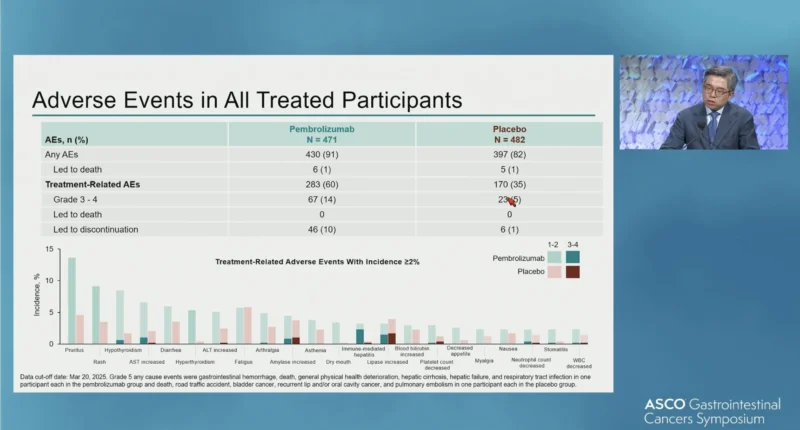
Treatment-related adverse events were more frequent in the pembrolizumab arm. Grade 3–4 treatment-related adverse events occurred in 14% of patients receiving pembrolizumab compared with 5% of those receiving placebo. Treatment-related adverse events leading to discontinuation were reported in 10% of patients in the pembrolizumab group and 1%in the placebo group. No treatment-related deaths were observed, and no new safety signals were identified for pembrolizumab monotherapy.
Conclusion
The phase III KEYNOTE-937 trial showed that adjuvant pembrolizumab did not improve recurrence-free survival compared with placebo in patients with hepatocellular carcinoma who achieved complete radiologic response after surgical resection or local ablation. No benefit was observed in overall survival or distant metastases-free survival, and treatment was associated with higher rates of high-grade adverse events.
These findings indicate that adjuvant pembrolizumab did not provide a clinically meaningful benefit in this curative-intent HCC population, as the primary recurrence-free survival endpoint was not met and no advantage was observed in secondary efficacy outcomes.
Read full abstract here.


