Immune checkpoint blockade (ICB) benefits only a subset of patients with head and neck squamous cell carcinoma (HNSCC), and commonly used biomarkers (PD-L1, TMB) remain imperfect in this setting. This study asks whether intratumoral bacterial biomass—not just “which species are present,” but how much bacteria is in the tumor—helps explain resistance to ICB.
Methods and Study Design
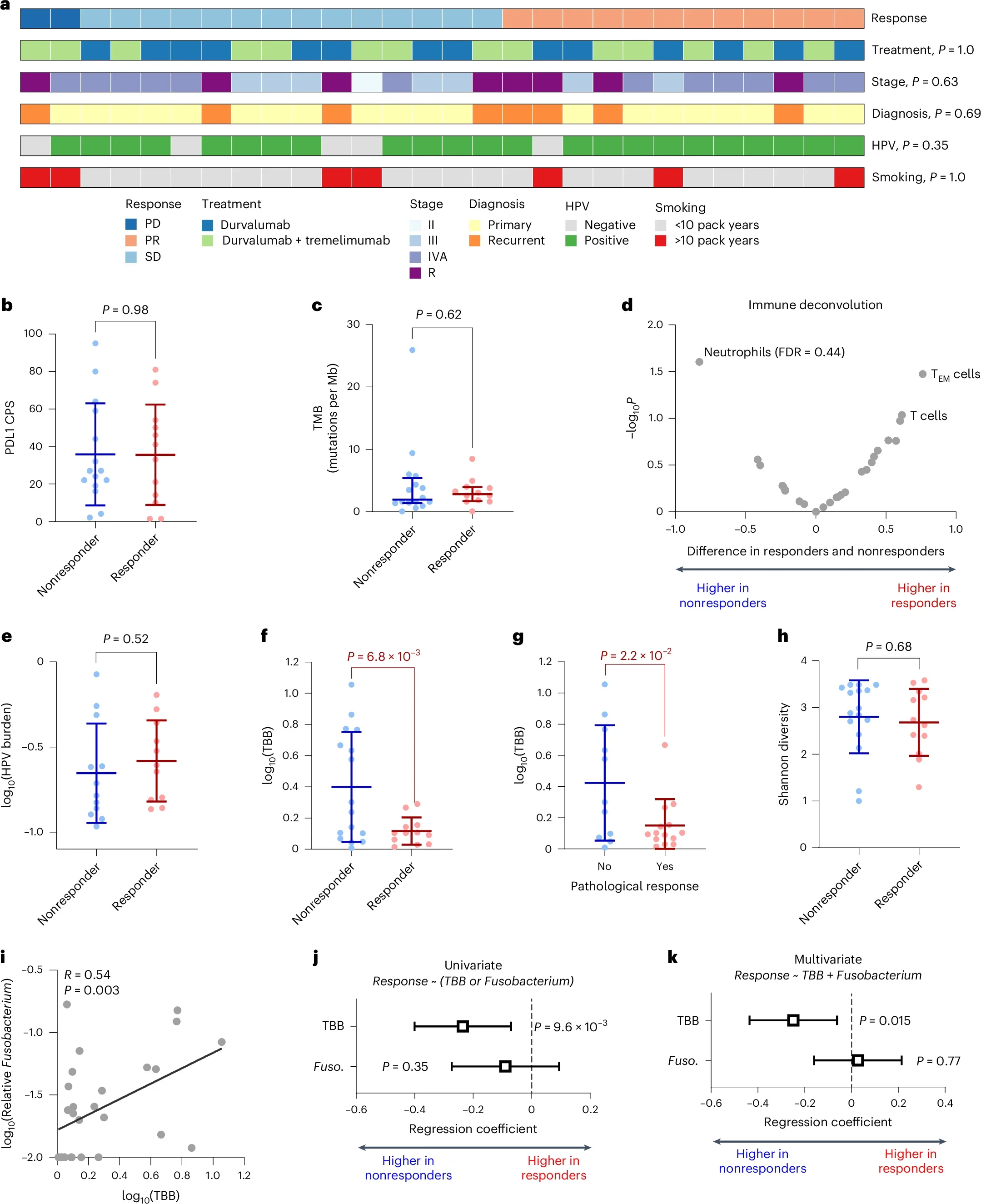
In the clinical discovery phase, the investigators focused on resectable oropharyngeal HNSCC treated on the CIAO neoadjuvant trial, where patients received durvalumab (anti–PD-L1) either alone or combined with tremelimumab (anti–CTLA-4) prior to surgery. From pretreatment and on-treatment tumor sequencing datasets, they defined their primary microbial feature—tumor bacteria burden (TBB)—as the number of bacterial reads per million human reads, aiming to quantify total intratumoral bacterial load rather than emphasizing any single taxon or relative microbial composition.
To test whether this signal was generalizable and not an artifact of a single cohort or assay, they then validated TBB across independent HNSCC datasets and broader multi-cancer cohorts, including cohorts in which prior work had emphasized specific organisms (such as Fusobacterium) in relation to ICB outcomes. Alongside sequencing-based validation, they used orthogonal measurement strategies—including approaches aligned with 16S-based profiling, qPCR correlation frameworks, and in situ methods—to support that sequencing-derived estimates of intratumoral bacterial abundance tracked with alternative bacterial detection/quantification techniques.
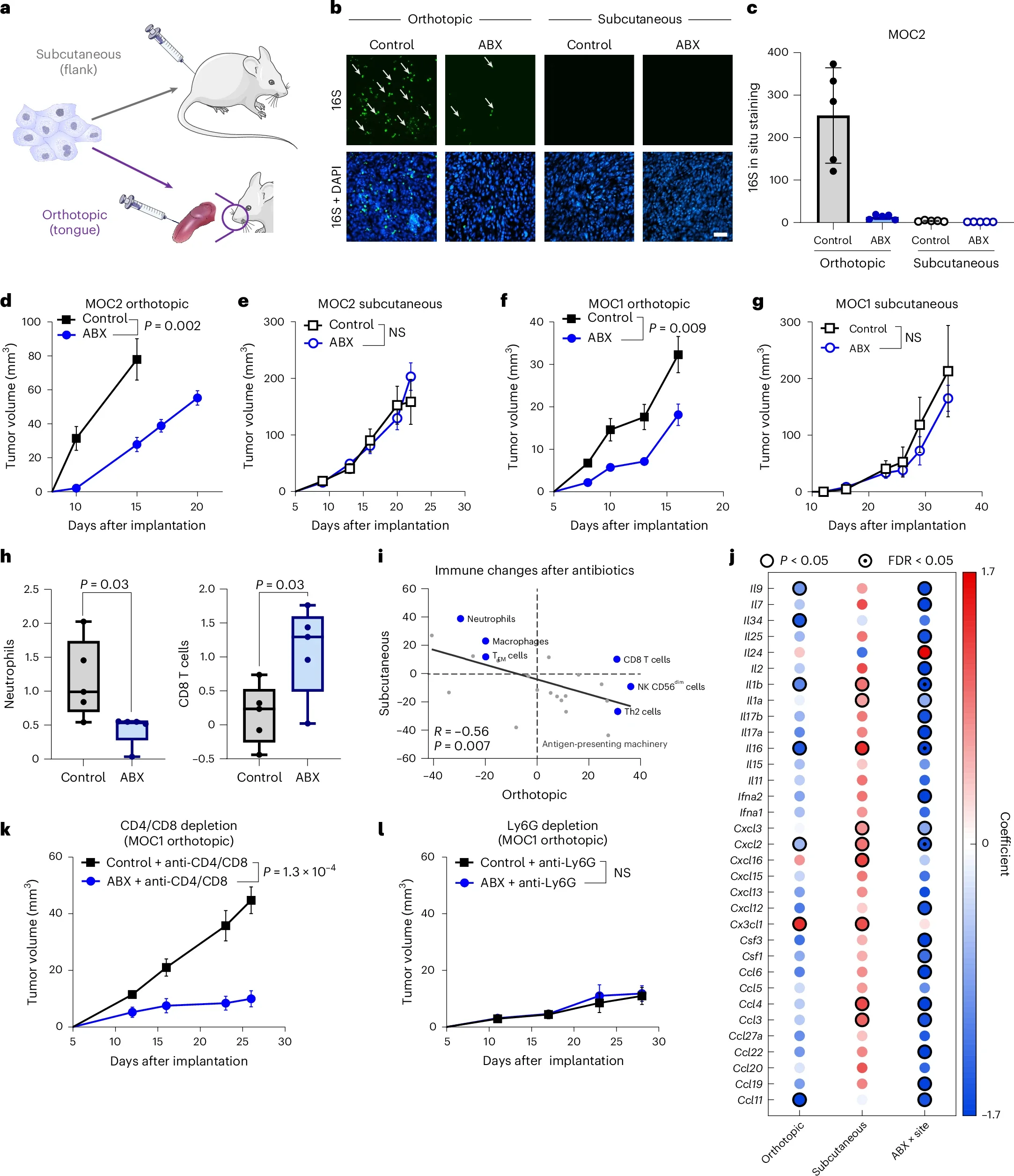
Finally, to move beyond association toward mechanism, they performed in vivo experiments in orthotopic head and neck cancer mouse models that naturally accumulate intratumoral bacteria at mucosal sites. In these models, they manipulated bacterial load in two directions: depleting intratumoral bacteria using a broad-spectrum antibiotic cocktail, and increasing intratumoral bacterial burden via oral inoculation with multiple bacterial taxa. After establishing these perturbations, they evaluated downstream immune and tumor effects, including sensitivity to anti–PD-L1 therapy, to directly test whether altering intratumoral bacterial abundance could causally shift the tumor immune microenvironment and immunotherapy response.
Intratumoral bacterial burden
Results
Within the CIAO neoadjuvant cohort, tumor bacterial burden (TBB) emerged as the most informative predictor of response to immune checkpoint blockade. Patients who achieved clinical responses consistently harbored lower levels of intratumoral bacteria, whereas established biomarkers such as PD-L1 combined positive score (CPS) and tumor mutational burden (TMB) showed no significant association with treatment response in this dataset. This positioned TBB as a dominant discriminator of benefit in this setting.
Importantly, the observed effect reflected overall bacterial load rather than the presence of a specific microorganism. Although the relative abundance of Fusobacterium correlated with TBB, it did not retain independent predictive value once bacterial burden was included in multivariable models. These analyses reinforced the concept that bacterial biomass itself—rather than taxonomic identity—is the key driver of the association with immunotherapy response.
High intratumoral bacterial burden was further linked to a distinct pattern of immune remodeling within the tumor microenvironment. Tumors with elevated TBB demonstrated features of immune suppression, characterized by enrichment of neutrophils alongside depletion of T cells and other adaptive immune populations, resulting in a higher intratumoral neutrophil-to-lymphocyte–like signal. This immune contexture aligned with reduced sensitivity to checkpoint blockade.
Causal evidence for this relationship was provided by in vivo experiments. In orthotopic HNSCC mouse models, pharmacologic depletion of intratumoral bacteria led to measurable changes in immune composition and tumor behavior, consistent with a shift toward a more permissive antitumor immune state. Conversely, augmenting intratumoral bacterial burden—through oral administration of multiple, distinct bacterial taxa—was sufficient to induce resistance to anti–PD-L1 therapy. Notably, this resistance phenotype was observed across different bacterial compositions, indicating that the effect is not species-specific, but rather driven by the magnitude of bacterial accumulation within the tumor.
Intratumoral bacterial burden
Insights
- This work reframes the intratumoral microbiome in mucosal tumors like HNSCC as a quantitative immune-modulator: bacterial accumulation appears to push tumors toward neutrophil-driven immunosuppression and away from effective adaptive antitumor immunity—creating a biologic state where ICB is less likely to work.
- Translationally, TBB could function as a stratification biomarker for neoadjuvant ICB decision-making (and possibly escalation/de-escalation strategies), especially where PD-L1/TMB are ambiguous.
Key Takeaway Messages
- High intratumoral bacterial burden predicts ICB resistance in HNSCC and holds up across cohorts.
- The dominant effect appears load-dependent, with resistance reproducible across multiple bacterial taxa.
- High TBB tumors show a consistent immune pattern: ↑ neutrophils / ↓ T cells, aligning with a suppressive microenvironment.
- The findings support clinical testing of bacteria-targeting strategies (carefully designed to avoid harming beneficial gut–immune interactions), and relevant interventional trials are emerging (e.g., antibiotics/chlorhexidine approaches aimed at reducing tumor microbial load).
Conclusion
This Nature Cancer 2026 study positions intratumoral bacterial biomass (TBB) as both a mechanistic driver of immunosuppression and a clinically actionable biomarker for immunotherapy resistance in HNSCC, with compelling human-to-mouse-to-therapy evidence linking “more bacteria in the tumor” to “less benefit from PD-(L)1 blockade.”
Read all article here


