At ASCO GI 2026, Kohei Shitara presented updated results from cohort 4 of the ILUSTRO trial, a phase II study evaluating zolbetuximab in combination with mFOLFOX6 and nivolumab as first-line therapy for patients with CLDN18.2-positive, HER2-negative locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma. The study explores whether adding PD-1 blockade to zolbetuximab-based chemotherapy can further improve outcomes in this biomarker-selected population.
Title: Phase 2 ILUSTRO trial of 1L zolbetuximab plus mFOLFOX6 and nivolumab in patients with CLDN18.2+ locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma.
Authors: Kohei Shitara, Hirokazu Shoji, Nicola Fazio, Sara Lonardi, Keun-Wook Lee, Li-Yuan Bai, Kensei Yamaguchi, Jean-Philippe Metges, Gianluca Masi, Denis M. Smith, Tae-Yong Kim, Maria Matsangou, Archita Shrivastava, Miaomai Zhou, Aziz Zaanan, and Samuel J. Klempner.
Methods
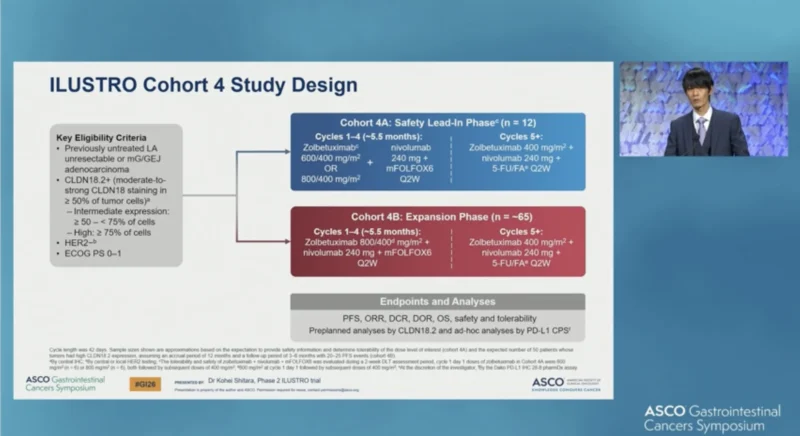
ILUSTRO is a multicohort phase II trial. Cohort 4 enrolled patients with HER2-negative locally advanced unresectable or metastatic gastric/GEJ adenocarcinoma expressing CLDN18.2, defined as moderate-to-strong membranous staining by immunohistochemistry in at least 50% of tumor cells, with a prespecified subgroup of high CLDN18.2 expression (≥75%). Prior systemic therapy for advanced disease was not permitted.
Cohort 4A established the tolerable dose of the triplet, followed by cohort 4B expansion. Patients received zolbetuximab 800/400 mg/m², nivolumab 240 mg, and mFOLFOX6 every two weeks for cycles 1–4 (~5.5 months), followed by zolbetuximab 400 mg/m², nivolumab 240 mg, and 5-FU/folinic acid every two weeks from cycle 5 onward. In Cohort 4A, zolbetuximab was evaluated at 600/400 mg/m² or 800/400 mg/m² to establish the tolerable dose for expansion.
Endpoints
Efficacy endpoints included progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), duration of response (DoR), and overall survival (OS). Safety and tolerability were also assessed.

Read More about Zolbetuximab (Vlyoy): Uses in Cancer, Side Effects, Dosages, Expectations on OncoDaily.
Results
In the Cohort 4B expansion, median PFS with zolbetuximab plus chemotherapy and nivolumab was 14.8 months (95% CI 8.3–not estimable), with 31 of 71 patients (43.7%) experiencing a PFS event at the time of analysis.
When stratified by CLDN18.2 expression, outcomes favored patients with high expression. Median PFS was 18.0 months (95% CI 11.1–NE) in the CLDN18.2-high subgroup (events 23/59; 39.0%), compared with 6.7 months (95% CI 3.0–NE) in patients with intermediate CLDN18.2 expression (events 7/11; 63.6%).
Within the CLDN18.2-high population, PD-L1 expression further differentiated outcomes. Median PFS reached 23.6 months in patients with PD-L1 CPS ≥1 (events 13/36; 36.1%), compared with 12.1 months in those with CPS <1(events 9/21; 42.9%).
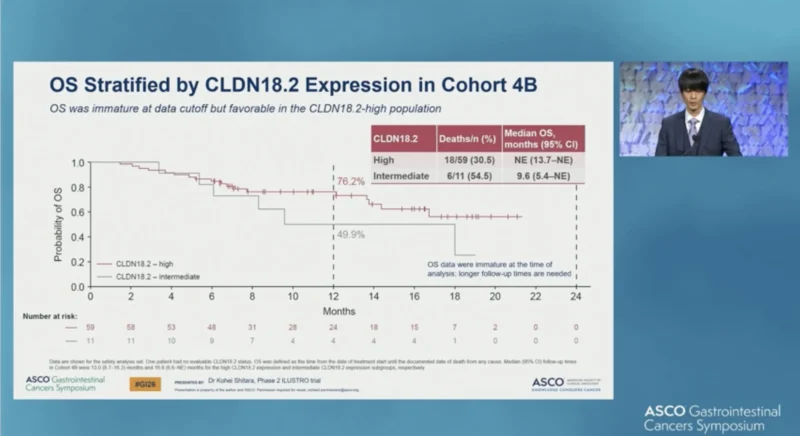
Overall survival (OS) data were immature at the time of analysis. Median OS was not estimable in the CLDN18.2-high subgroup (deaths 18/59; 30.5%), while patients with intermediate CLDN18.2 expression had a median OS of 9.6 months(95% CI 5.4–NE; deaths 6/11; 54.5%).
Objective responses were frequent and durable. Among patients with measurable disease, the objective response rate (ORR) was 62.1% overall (36/58; 95% CI 48.4–74.5) and 68.1% in the CLDN18.2-high subgroup (32/47; 95% CI 52.9–80.9), compared with 40.0% in patients with intermediate CLDN18.2 expression (4/10; 95% CI 12.2–73.8). Median duration of response was 19.1 months overall (95% CI 10.8–NE), was not estimable in the CLDN18.2-high subgroup, and was 19.1 months (95% CI 3.5–NE) in the intermediate subgroup.
Across Cohorts 4A and 4B (N = 77), the safety profile was manageable with no unexpected signals. Treatment-emergent adverse events led to discontinuation of zolbetuximab in 5.2% of patients and nivolumab in 7.8%, while discontinuation of any study drug occurred in 49.4%, largely driven by chemotherapy-related toxicities. Nausea and decreased appetite were the most frequently reported adverse events.
Conclusion
First-line treatment with zolbetuximab plus mFOLFOX6 and nivolumab demonstrated promising antitumor activity in patients with CLDN18.2-positive, HER2-negative advanced gastric or GEJ adenocarcinoma, with particularly favorable outcomes in those with high CLDN18.2 expression.
The safety profile was manageable and aligned with prior experience. These results support continued phase III development, including the phase III LUCERNA trial, which is evaluating zolbetuximab plus pembrolizumab and chemotherapy as first-line treatment in patients with CLDN18.2-positive, HER2-negative, PD-L1–positive locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma.
You can read the full abstract here.


