Fresh from the podium at ESTRO 2025, Dr. Thomas Brunner has just presented the latest data from the ESOPEC trial, offering brand-new insights into the ongoing debate over optimal perioperative treatment for esophageal and gastroesophageal junction (GEJ) adenocarcinoma. As previously covered on OncoDaily, the ESOPEC trial remains the largest randomized study in this space, comparing perioperative FLOT chemotherapy with neoadjuvant chemoradiotherapy (CROSS protocol). These just-released findings provide the most up-to-date evidence on survival outcomes, treatment-related toxicity, and patient selection—potentially influencing clinical practice in real time.
Background of ESOPEC trial
Esophageal cancer affects over 510,000 people annually and remains a leading cause of cancer mortality worldwide, with over 445,000 deaths per year. The incidence of esophageal adenocarcinoma and esophagogastric junction tumors continues to rise, particularly in high-income countries.Radical esophagectomy is the curative standard for nonmetastatic resectable esophageal adenocarcinoma, but recurrence rates remain high, with 5-year survival rates rarely exceeding 35%. Two multimodal treatment strategies have shown survival benefits:
- Preoperative chemoradiotherapy (CROSS protocol): Weekly carboplatin and paclitaxel with 41.4 Gy radiotherapy, followed by surgery.
- Perioperative chemotherapy (FLOT regimen): Four cycles of fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) before and after surgery.
The ESOPEC trial was designed to directly compare these two approaches in terms of overall survival.
Methods
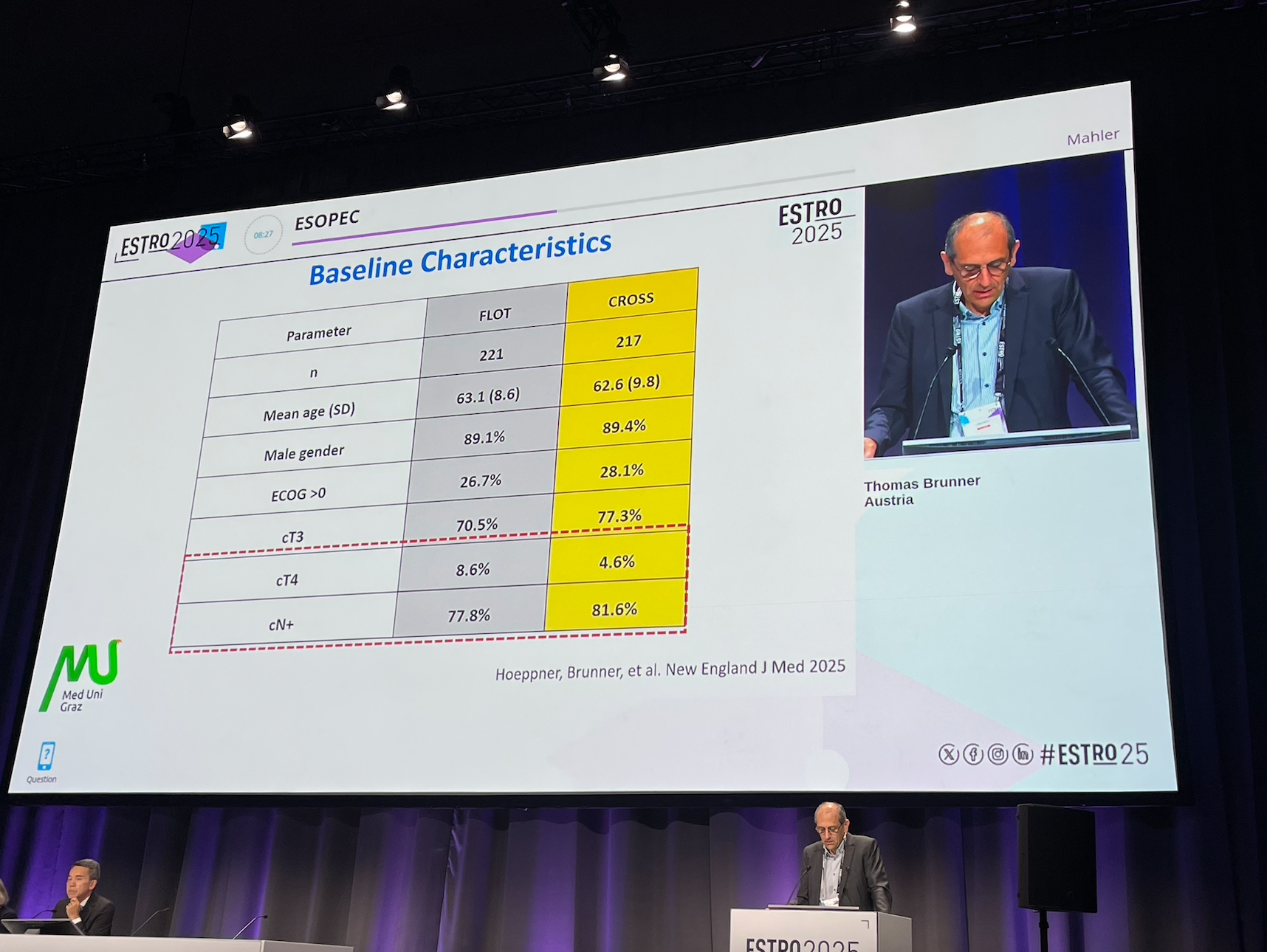
The ESOPEC trial was a phase 3, multicenter, randomized controlled trial conducted across 25 centers in Germany. Patients with histologically confirmed resectable esophageal adenocarcinoma (UICC clinical stage cT1 cN+, cT2-4a cN+, or cT2-4a cN0) were randomized 1:1 to receive:
- Perioperative FLOT chemotherapy: Four preoperative and four postoperative cycles (fluorouracil, leucovorin, oxaliplatin, docetaxel).
- Preoperative chemoradiotherapy (CROSS regimen): Weekly carboplatin and paclitaxel with 41.4 Gy radiotherapy followed by surgery.
Primary endpoint: Overall survival (OS) Secondary endpoints: Progression-free survival (PFS), recurrence rates, surgical outcomes, adverse events.
Key Results
The ESOPEC trial randomized a total of 438 patients with resectable esophageal or gastroesophageal junction (GEJ) adenocarcinoma to either perioperative FLOT chemotherapy (n=221) or neoadjuvant chemoradiotherapy using the CROSS protocol (n=217). The primary endpoint was overall survival, with progression-free survival, recurrence patterns, and safety outcomes assessed as secondary endpoints.
Treatment Delivery and Compliance
-
Preoperative therapy completion was high in both groups:
-
In the FLOT arm, 97% of patients received at least three of four planned cycles.
-
In the CROSS arm, 94% completed four out of five chemotherapy cycles during concurrent chemoradiation.
-
-
Adjuvant chemotherapy (postoperative FLOT) was delivered in 56% of patients, consistent with the known challenges of postoperative recovery.
-
Radiotherapy quality assurance (RTQA) compliance was strong at 92% in both arms, supporting the consistency of radiotherapy delivery across participating centers.
Survival Outcomes
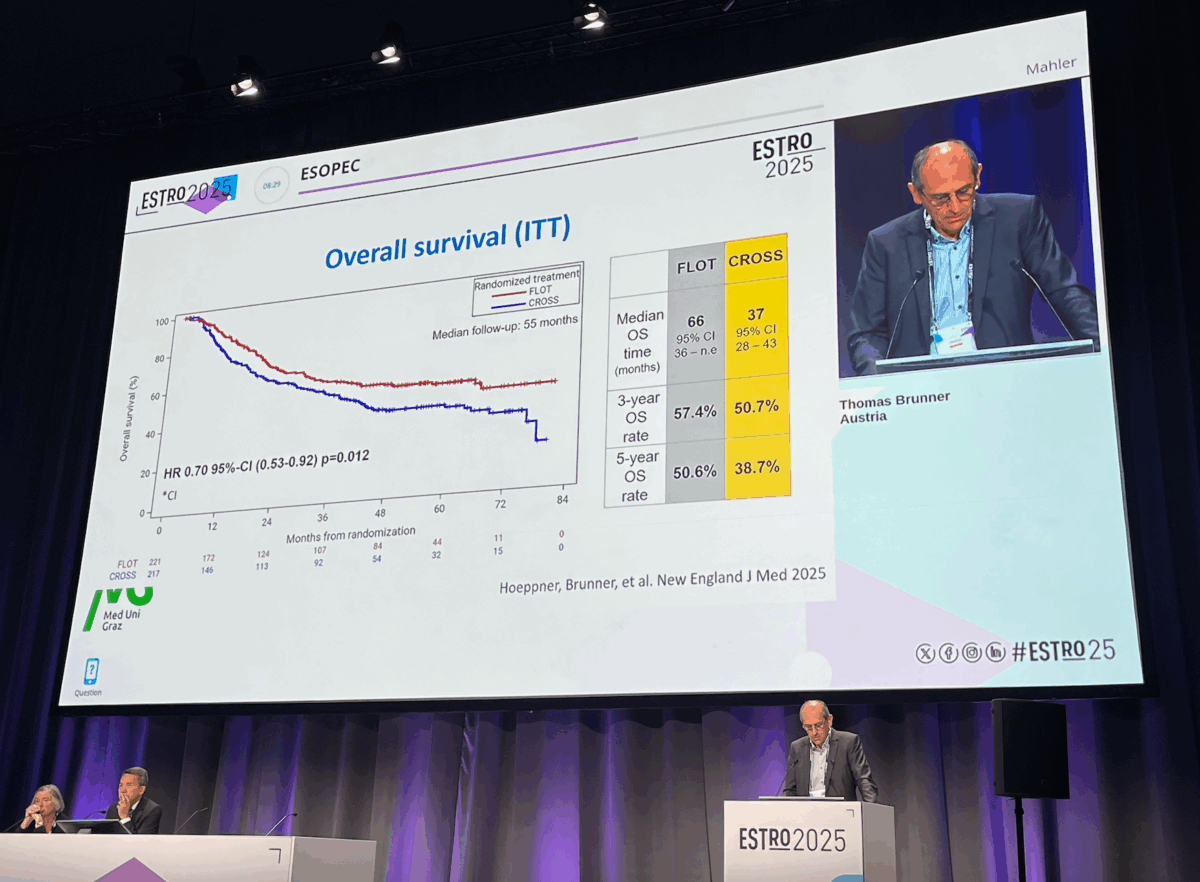
FLOT demonstrated a statistically significant advantage in both overall and progression-free survival:
-
3-year overall survival (OS):
-
57% with FLOT
-
50% with CROSS
-
-
Median OS:
-
66 months in the FLOT group
-
37 months in the CROSS group
-
-
Hazard ratio for OS: 0.77, favoring FLOT, though slightly above the predefined threshold (HR 0.645) in the statistical plan.
Progression-free survival also favored the FLOT regimen:
-
Median PFS:
-
60 months with FLOT
-
38 months with CROSS
-
-
While specific 3-year PFS rates were not disclosed, the survival curve separation consistently favored FLOT.
Pathological Response
-
Complete pathological response (pCR, ypT0N0):
-
FLOT: 17%
-
CROSS: 10%
-
-
Major pathological response (≥90% tumor regression):
-
CROSS: 53%
-
FLOT: 43%
-
This contrast underscores the strength of CROSS in achieving local tumor control, while FLOT offered broader systemic benefit as evidenced by survival outcomes.
Recurrence Patterns
Distant recurrence remained the predominant mode of failure, with notable differences between arms:
-
Isolated distant relapse:
-
37% in CROSS
-
21% in FLOT
-
-
Isolated locoregional recurrence:
-
4% in CROSS
-
8% in FLOT
-
These findings highlight the superior systemic control of FLOT, while CROSS provided more robust locoregional control, likely attributable to its radiotherapy component.
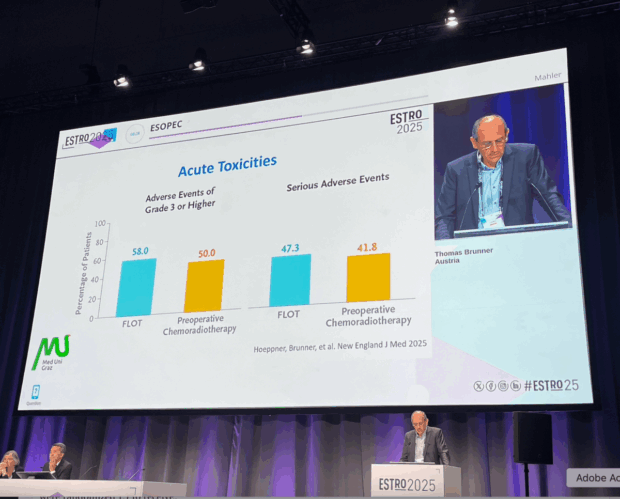
Toxicity and Safety
-
Grade ≥3 adverse events occurred more frequently in the FLOT group, though differences were not statistically significant.
-
Serious adverse events were slightly lower in the CROSS group.
-
Postoperative morbidity was comparable across both treatment arms, with no unexpected safety signals reported.
Key Takeaways
- CRT still plays a role in the multidisciplinary care of oesophageal and gastric adenocarcinoma
- Still standard if FLOT not tolerated (no good evidence for FLO)
- Checkmate 577 overal survival data (eagerly) awaited
- More potent forms of the chermorads backbone: CALGB 80803
(FOLFOX and 50.4 Gy in 28#) - Earlier stages of EAC as in ESOPEC for organ preservation after induction chemotherapy
Summary by Sona Karamyan, MD, Research Analyst – Medical Intelligence Department of OncoDaily
Read ESTRO 2025 Live Coverage by OncoDaily


