The APOLLO trial marks a significant advancement in the treatment landscape of unresectable hepatocellular carcinoma (HCC), particularly in regions where hepatitis B virus (HBV) is the predominant cause. While immune checkpoint inhibitors (ICIs) have improved response durability, previous ICI-based combinations have not consistently translated into overall survival benefit.
Penpulimab, a novel PD-1 inhibitor with engineered Fc silencing to reduce immune-related toxicity, and anlotinib, a multitargeted tyrosine kinase inhibitor (TKI) with anti-angiogenic and immunomodulatory properties, showed encouraging early-phase results. The APOLLO study was designed to evaluate whether this combination could outperform sorafenib in improving survival outcomes among Chinese patients with advanced, treatment-naïve HCC.
Title: Anlotinib plus penpulimab versus sorafenib in the first-line treatment of unresectable hepatocellular carcinoma (APOLLO): a randomised, controlled, phase 3 trial
Authors: Prof Jian Zhou, MD, Prof Li Bai, MDb, Prof Jia Luo, MD, Prof Yuxian Bai, MD, Prof Yaozhen Pan, MD, Prof Xinrong Yang, MD, Prof Yufeng Gao, MD, Prof Rongshu Shi, BS, Wenhua Zhang, BS, Prof Jinfang Zheng, MD, Xiangdong Hua, MD, Prof Aibing Xu, BS, Prof Sheng Hu, MD, Prof Feng Zhang, MD, Prof Xiaojun Yang, MD, Prof Mingxu, MD, Rui Wang, MD, Prof Jie Ma, MD, Prof Weidong Jia, MD, Prof Dongmei Quan, MD, Prof Chuang Peng, MD, Prof Wei Yang, MD, Prof Guowen Yin, MD, Yue Qi, MD, Prof Guifang Zhang, BS, Prof Xilin Du, MD, Prof Xiaorong Mao, MD, Prof Zhiqiang Meng, MD, Prof Shunchang Jiao, MD, Prof Jia Fan, MD
Published in Lancet Oncology, May 2025
Background
Hepatocellular carcinoma (HCC), the most prevalent form of primary liver cancer, is the third leading cause of cancer-related mortality globally. In China, where hepatitis B virus (HBV) is endemic, HBV-related HCC exhibits aggressive clinical features such as vascular invasion and elevated α-fetoprotein (AFP) levels. While immune checkpoint inhibitors (ICIs) have revolutionized the treatment of advanced HCC, many ICI-based combination strategies—especially those pairing ICIs with multi-target tyrosine kinase inhibitors (TKIs)—have failed to show overall survival (OS) benefit over sorafenib, the historical standard of care. Penpulimab, a novel PD-1 inhibitor engineered to minimize immune-related toxicities, and anlotinib, a multitarget TKI with antiangiogenic and immunomodulatory activity, showed encouraging efficacy in earlier studies. The phase 3 APOLLO trial evaluated whether this combination could improve survival outcomes compared to sorafenib in Chinese patients with unresectable HCC.
Methods and Study Design
APOLLO was a randomized, open-label, multicenter phase 3 trial conducted at 79 sites across China. Patients with unresectable, treatment-naïve HCC were randomly assigned (2:1) to receive either anlotinib plus penpulimab or sorafenib.
Patients aged 18–75 years with Barcelona Clinic Liver Cancer (BCLC) stage B (not amenable to local therapy) or stage C HCC, Child-Pugh A/B7 liver function, ECOG 0–1, and measurable disease per RECIST 1.1 were eligible. HBV-infected patients had to be on or eligible for antiviral therapy with controlled viral load.
The experimental arm received anlotinib (10 mg orally, days 1–14) plus penpulimab (200 mg IV, day 1 of a 21-day cycle). The control arm received sorafenib (400 mg orally twice daily). Treatment continued until disease progression, unacceptable toxicity, or withdrawal.
Results
The APOLLO trial demonstrated that the combination of anlotinib and penpulimab provides clinically meaningful improvements in progression-free and overall survival over sorafenib in Chinese patients with unresectable hepatocellular carcinoma. With a favorable efficacy-to-safety balance, this regimen offers a promising new first-line treatment alternative. Future studies may confirm its role in broader global populations and further explore its place in HCC treatment algorithms.
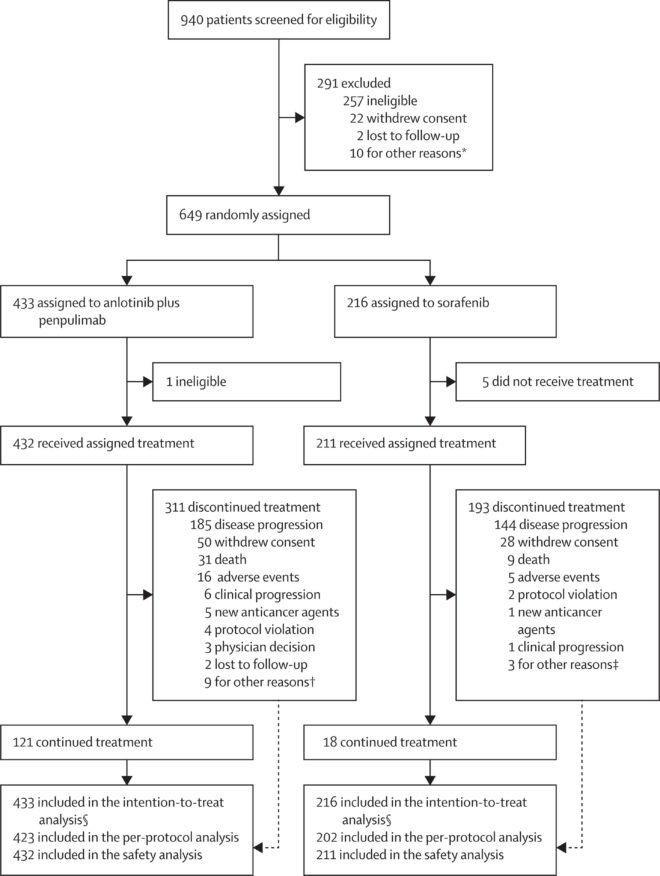
A total of 649 patients were randomized in the APOLLO trial, with 433 patients assigned to receive the combination of anlotinib and penpulimab and 216 patients assigned to sorafenib. The study population had a median age of 57 years, and 85% of participants were male. Most patients (84%) had hepatitis B virus (HBV)-related HCC, and 80% presented with either macrovascular invasion or extrahepatic metastasis.
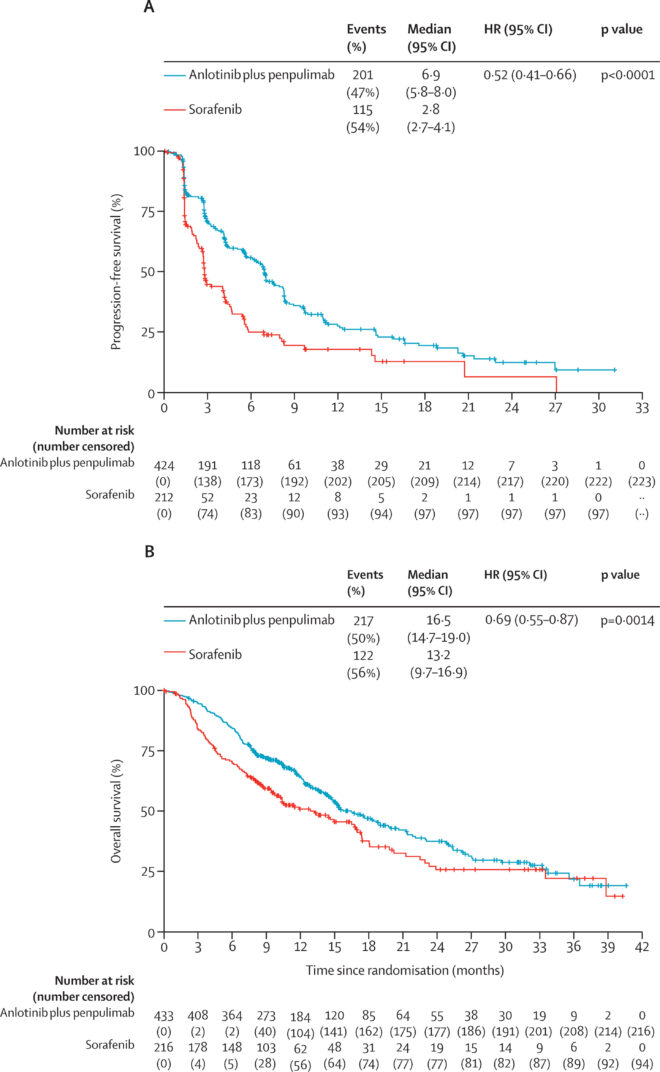
- Progression-free survival (PFS) was significantly improved with the combination therapy.
- Median PFS was 6.9 months (95% CI: 5.8–8.0) for anlotinib–penpulimab vs 2.8 months (95% CI: 2.7–4.1) for sorafenib.
- This corresponded to a hazard ratio (HR) of 0.52 (95% CI: 0.41–0.66; p<0.0001), representing a 48% reduction in the risk of progression or death.
- The 12-month PFS rate was 27.4% in the combination arm vs 17.8% in the sorafenib group.
- Median OS was 16.5 months (95% CI: 14.7–19.0) in the anlotinib–penpulimab group compared to 13.2 months (95% CI: 9.7–16.9) in the sorafenib group.
- The OS benefit was statistically significant (HR: 0.69, 95% CI: 0.55–0.87; p=0.0014).
- At 24 months, the OS rate was 37.5% for the combination arm vs 25.7% for sorafenib.
- The objective response rate (ORR) by investigator assessment using RECIST 1.1 was significantly higher in the experimental arm: ORR was 21% (95% CI: 17–25) with anlotinib–penpulimab, compared to 7% (95% CI: 4–12) with sorafenib (p<0.0001).
- The disease control rate (DCR), including complete response, partial response, and stable disease, was: 73% (95% CI: 68–77) in the combination group and 56% (95% CI: 49–62) in the sorafenib group (p<0.0001)
- The median time to response was 2.7 months (IQR: 1.4–4.2) with the combination and 1.5 months (IQR: 1.4–4.0) with sorafenib.
- The median duration of response was 16.2 months (95% CI: 11.2–not evaluable) for anlotinib–penpulimab and 16.8 months (95% CI: 5.7–not evaluable) for sorafenib.
The safety profile was manageable: Grade ≥3 treatment-related adverse events (TRAEs) occurred in 50% of patients in the anlotinib–penpulimab group and 48% in the sorafenib group.
The most common grade ≥3 TRAEs in the combination arm were:
- Hypertension (17%)
- Thrombocytopenia (9%)
- Neutropenia (6%)
- Leukopenia (6%)
- Palmar-plantar erythrodysesthesia syndrome, a typical sorafenib-related toxicity, was more frequent in the sorafenib group (8%) compared to the combination group (2%).
Treatment discontinuation due to TRAEs occurred in:
- 9% of patients receiving anlotinib plus penpulimab
- 4% of those treated with sorafenib
After treatment discontinuation, subsequent systemic therapy was administered to 37% of patients in the combination group and 44% in the sorafenib group.
Overall, the combination of anlotinib plus penpulimab not only extended survival and delayed disease progression but also achieved higher tumor response rates compared to sorafenib, with a manageable safety profile consistent with known effects of TKIs and PD-1 inhibitors.
Key Findings
- Anlotinib plus penpulimab significantly prolonged progression-free survival compared to sorafenib (6.9 vs. 2.8 months; HR: 0.52; p<0.0001).
- The overall survival benefit was also statistically significant (16.5 vs. 13.2 months; HR: 0.69; p=0.0014).
- The combination therapy yielded a higher objective response rate (21% vs. 7%) and disease control rate (73% vs. 56%).
- Median duration of response exceeded 16 months in both groups, suggesting durable benefit among responders.
- The safety profile was manageable, with slightly higher discontinuation in the experimental arm but no unexpected toxicities.
Key Takeaway Messages
- Anlotinib plus penpulimab significantly improved both progression-free and overall survival compared to sorafenib in treatment-naïve unresectable HCC.
- The combination was associated with a higher objective response rate (21% vs. 7%) and prolonged PFS (6.9 vs. 2.8 months).
- Toxicity was acceptable and consistent with known profiles; immune-related events were manageable due to penpulimab’s engineered Fc silencing.
- These findings support the potential of this ICI–TKI combination as a first-line option in advanced HCC.
You can read the full article here.
Written by Sona Karamyan,MD