
Agenus Enters New Era With $141M Zydus Deal Signaling Strategic Transformation in Immuno-Oncology
Agenus Inc. (Nasdaq: AGEN) is a U.S.-based biotechnology company specializing in immuno-oncology, focused on discovering and developing checkpoint inhibitors, cell therapies, and immune modulators to treat cancer. Known for its investigational drugs botensilimab (BOT) and balstilimab (BAL), Agenus is targeting some of the most treatment-resistant tumors in oncology today—including microsatellite-stable colorectal cancer (MSS CRC), hepatocellular carcinoma (HCC), sarcomas, and triple-negative breast cancer (TNBC).
Zydus Lifesciences Ltd., headquartered in India, is a multinational pharmaceutical company with over 27,000 employees and operations across 55 countries. As one of India’s largest life sciences companies, Zydus has deep experience in generics, biologics, and vaccine development, and is expanding its global presence in contract development and manufacturing (CDMO) for biologics.
A New Financial Engine: $141M Deal with Zydus
On June 3, 2025, Agenus revealed a multi-tiered strategic collaboration with Zydus Lifesciences, marking a critical shift in how the company funds and scales its most promising therapies. Under the agreement:
- $75 million upfront to Agenus for the transfer of biologics manufacturing assets in California.
- $50 million in contingent milestone payments tied to BOT/BAL production.
- $16 million equity investment from Zydus at $7.50 per share.
- Exclusive BOT/BAL rights in India and Sri Lanka granted to Zydus with a 5% royalty on net sales.
Zydus will repurpose the facilities as the base of its new U.S. BioCDMO business, while Agenus becomes its first customer, securing long-term manufacturing for BOT/BAL without owning operational risk. The partnership gives Agenus access to capital and infrastructure, while enabling Zydus to enter the U.S. biologics CDMO market with a flagship client.

Why This Deal Matters?
This isn’t just a capital injection—it’s a structural transformation. Agenus is effectively monetizing non-core assets while ensuring BLA-readiness for BOT/BAL through guaranteed manufacturing access. More than just survival capital, this deal offers strategic, non-dilutive funding that positions Agenus for late-stage clinical acceleration and eventual commercialization.
CEO Dr. Garo Armen described the partnership as a direct outcome of improving U.S.-India trade relations and a shared vision for global biopharma resilience:
“This agreement is an expression of confidence in the future of Agenus and in the regulatory environment of the United States.”
Zydus, with operations in 55 countries and over 27,000 employees, brings manufacturing depth and international reach. The deal also allows BOT/BAL to enter new markets like India and Sri Lanka, creating a broader clinical and commercial runway.
Agenus’s Strategic Shift and the Appointment of Dr. Richard Goldberg
Stepping out of retirement, Dr. Richard Goldberg brings four decades of experience in GI cancers to Agenus. Having served as director of the West Virginia University Cancer Institute, and previously holding key leadership roles at The Ohio State University’s James Cancer Hospital and UNC Lineberger Comprehensive Cancer Center, Goldberg’s arrival signals Agenus’s renewed commitment to pushing BOT/BAL forward toward regulatory approval.

Dr. Goldberg, expressing his commitment, remarked,
“Despite incremental treatment advances, patients and their families clamor daily for new and better treatments. They want more time, better time, and ultimately curative interventions.”
He sees the BOT/BAL combination as “among the most promising approaches on the horizon.” His leadership could be the catalyst needed to drive BOT/BAL through critical regulatory milestones.
What drugs are Botensilimab (BOT) and Balstilimab (BAL)?
BOT is a novel Fc-enhanced CTLA-4 inhibitor designed to activate both innate and adaptive immune responses, especially in “cold” tumors—traditionally resistant to standard immunotherapies. It primes and activates T cells, reduces intratumoral regulatory T cells, stimulates myeloid cells, and promotes durable memory responses.
BAL, Agenus’s PD-1 inhibitor, blocks the PD-1 receptor interaction with its ligands PD-L1 and PD-L2, enhancing T-cell activation and immune-mediated tumor destruction. Combined, BOT/BAL has demonstrated potent and durable anti-tumor activity across multiple challenging cancers, notably colorectal and hepatocellular carcinoma (HCC).
What Does the Latest Data Reveal?
In early 2025, Agenus revealed remarkable findings from several trials at the American Association for Cancer Research (AACR) Annual Meeting. The groundbreaking NEOASIS trial, for instance, demonstrated significant pathological responses in both microsatellite stable (MSS) and microsatellite unstable (MSI-H) solid tumors, including notoriously resistant triple-negative breast cancers (TNBC).
- NEOASIS Trial: Pathological complete response (pCR) rates reached an unprecedented 70% in MSI-H tumors and a noteworthy 20% in MSS tumors—demonstrating BOT/BAL’s efficacy beyond traditional responder groups.
- HCC Cohort: In patients with hepatocellular carcinoma who progressed after prior immunotherapies, BOT/BAL achieved a 17% overall response rate and an impressive 72% disease control rate. The median overall survival was an encouraging 12.3 months.
- Metastatic CRC Data: In a randomized Phase 2 trial involving MSS colorectal cancer patients previously resistant to standard therapies, BOT/BAL achieved a 19% overall response rate, while standard care showed 0%.
NEOASIS Study (Neoadjuvant Pan-Cancer Setting)
Led by Myriam Chalabi, MD PhD, this investigator-initiated study assessed BOT/BAL efficacy in early-stage solid tumors, both MMR-proficient (pMMR) and MMR-deficient (dMMR).
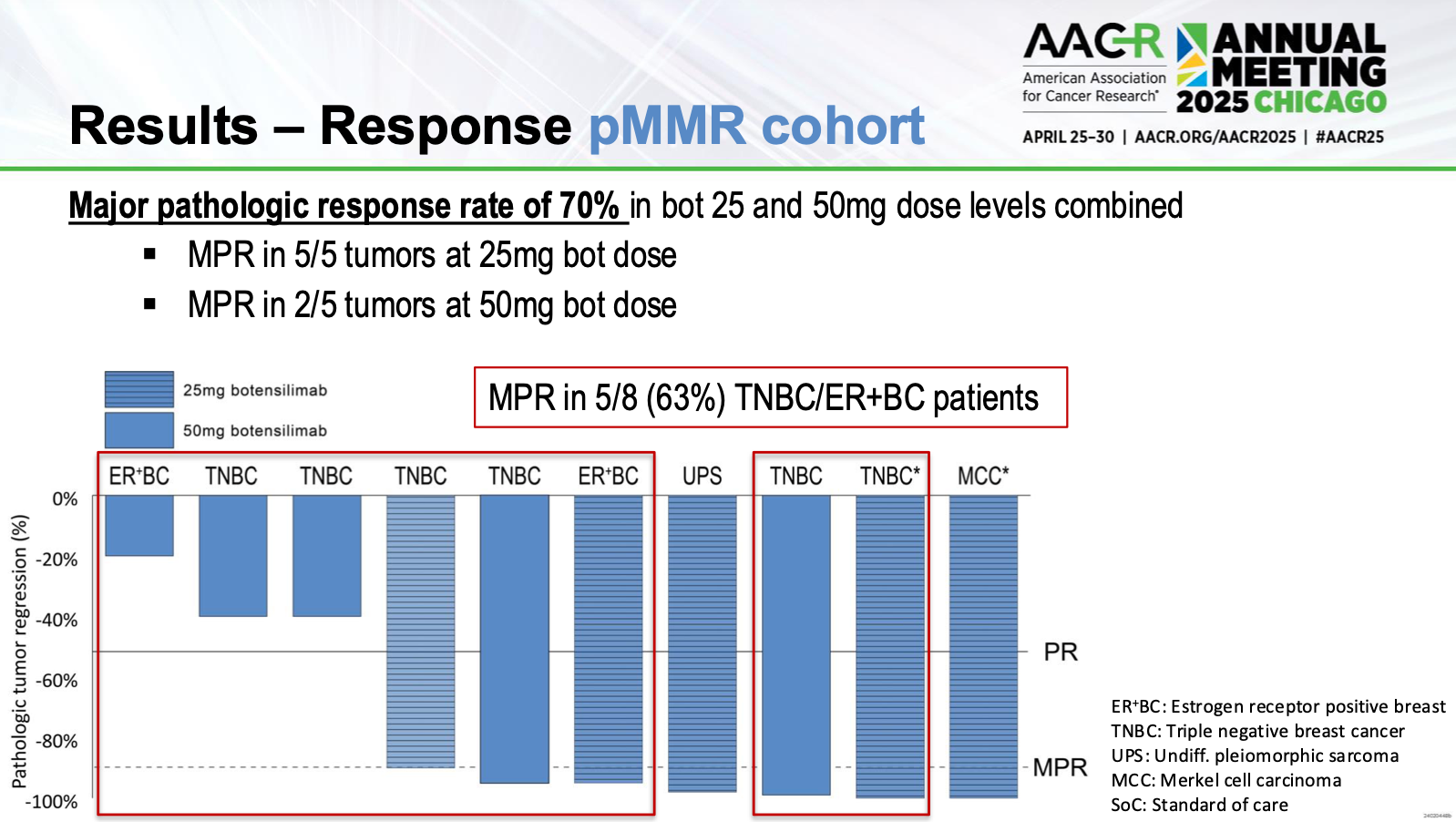
- Safety: At both 25mg and 50mg botensilimab doses, no significant delays or serious dose-limiting toxicities occurred. Mild and manageable immune-related adverse events were observed.
- Efficacy (pMMR cohort):
- Major pathological response (MPR): 70%
- Triple-negative breast cancer subset showed 63% MPR.
- Merkel cell carcinoma: Clear macroscopic regression with complete pathological response.
- Efficacy (dMMR cohort):
- 80% MPR, with 70% achieving pathological complete response (pCR)
- 100% pCR at the 50mg BOT dose.
Chalabi concluded that BOT/BAL effectively induces responses in traditionally immune-cold tumors, potentially broadening immunotherapy applications.
Phase 1 BOT/BAL in Relapsed/Refractory MSS CRC
This study evaluated BOT/BAL in heavily pretreated patients with relapsed or refractory MSS metastatic CRC. Results showed that patients without active liver metastases (a major driver of immunotherapy resistance) derived notable benefit, helping shape the design of Agenus’s ongoing Phase 2 trials.
- Overall response rate (ORR): 17%
- Disease control rate (DCR): 61%
- 12-month overall survival (OS): 69% in patients with non-liver metastases (n=77) and 30% in those with active liver metastases (n=24)
- Non-liver metastases subgroup: ORR: 22% and DCR: 73%
These findings helped refine the MSS CRC population most likely to benefit from BOT/BAL, setting the stage for the randomized Phase 2 trial that followed.
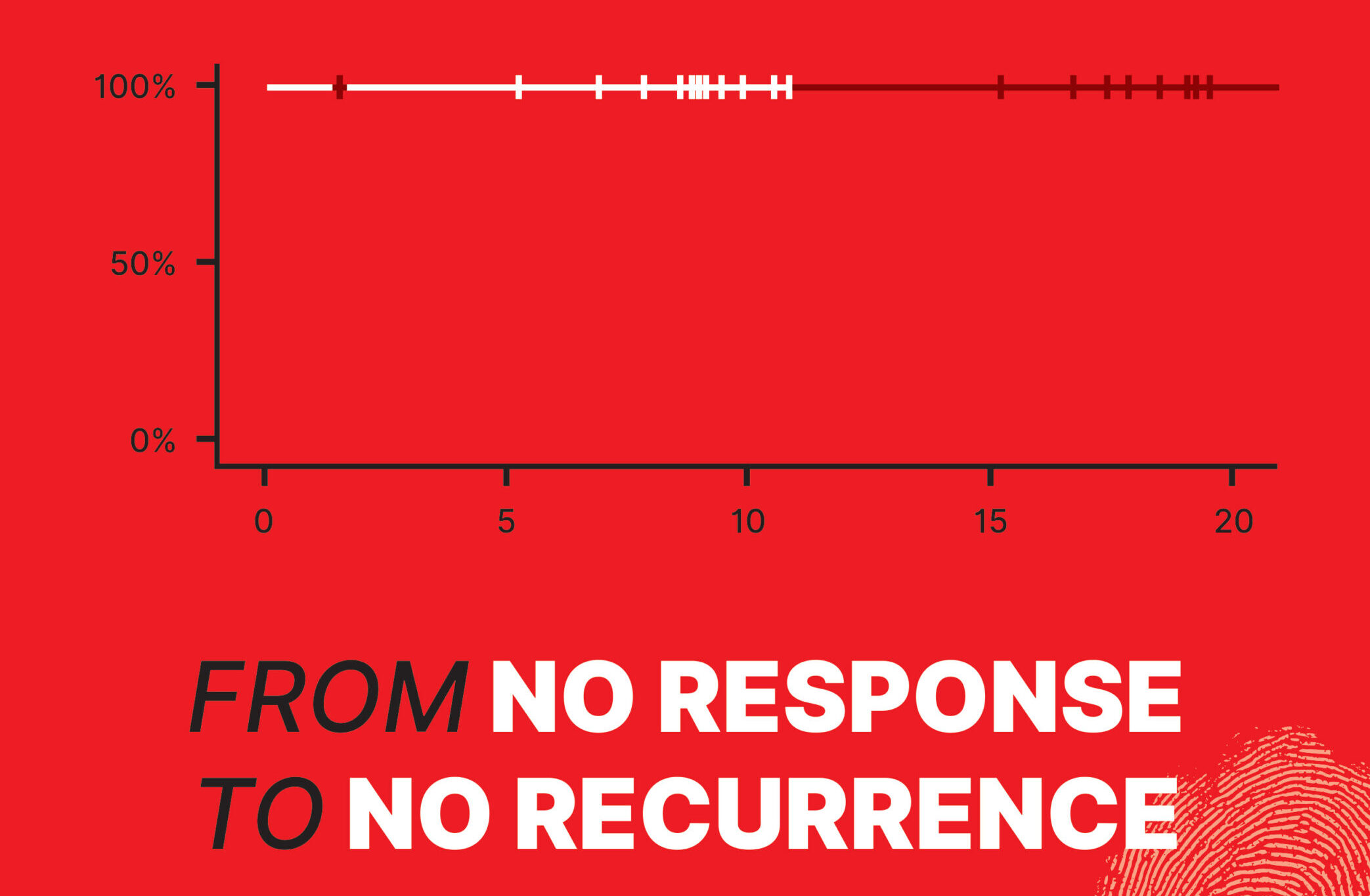
NEST Trials: No Recurrence with Dual Checkpoint Blockade in Neoadjuvant CRC
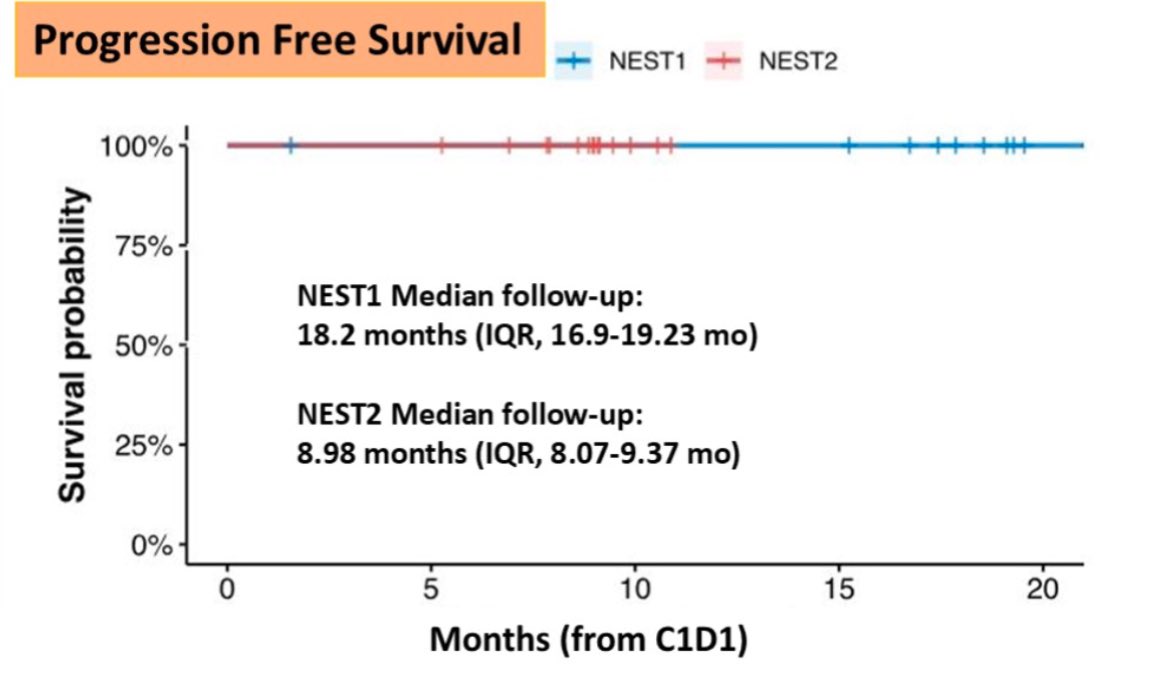
The NEST-1 and NEST-2 studies, investigator-initiated Phase 2 trials, evaluated neoadjuvant BOT/BAL in resectable colorectal cancer. Unlike previous efforts focused on metastatic disease, these trials brought checkpoint inhibition earlier—before surgery. Key Findings (presented at ASCO GI and ESMO GI):
- 100% ctDNA-negative status at median 18-month (NEST-1) and 9-month (NEST-2) follow-up
- No disease recurrence reported to date
- Major pathological response (MPR): Improved to 47% in MSS tumors in NEST-2
There were No grade 4–5 adverse events and No delays to surgery. The data underscore how early immunotherapy—especially in combination—may shift curative strategies in colorectal cancer toward organ preservation and reduced need for chemotherapy.
UNICORN Trial: BOT ± BAL in Resectable CRC
Led by Dr. Filippo Ghelardi and presented at ASCO GI 2025, the UNICORN trial explored short-course neoadjuvant therapy using BOT alone or in combination with BAL in patients with non-metastatic CRC.
Pathologic complete response (pCR):
- 93% in dMMR tumors
- 29% in pMMR tumors receiving BOT + BAL
Major pathological response (MPR): 36% in pMMR group
The addition of BAL significantly improved outcomes, especially in pMMR tumors, suggesting that even in traditionally resistant subtypes, immune priming via CTLA-4 blockade can be amplified through PD-1 inhibition.
Phase 1 BOT/BAL in Hepatocellular Carcinoma (HCC)
Dr. Anthony El-Khoueiry reported promising results for patients with advanced HCC previously unresponsive to first-line immunotherapies:
- Overall Response Rate (ORR): 17%
- Disease Control Rate (DCR): 72%
- Median Progression-Free Survival (PFS): 4.4 months
- Median Overall Survival (OS): 12.3 months
Safety profiles were consistent and manageable, encouraging further exploration in larger studies.
PD-1 Refractory Gastroesophageal Cancer: AgenT-797 + Bot + Bal with Chemotherapy (MSKCC Phase II)
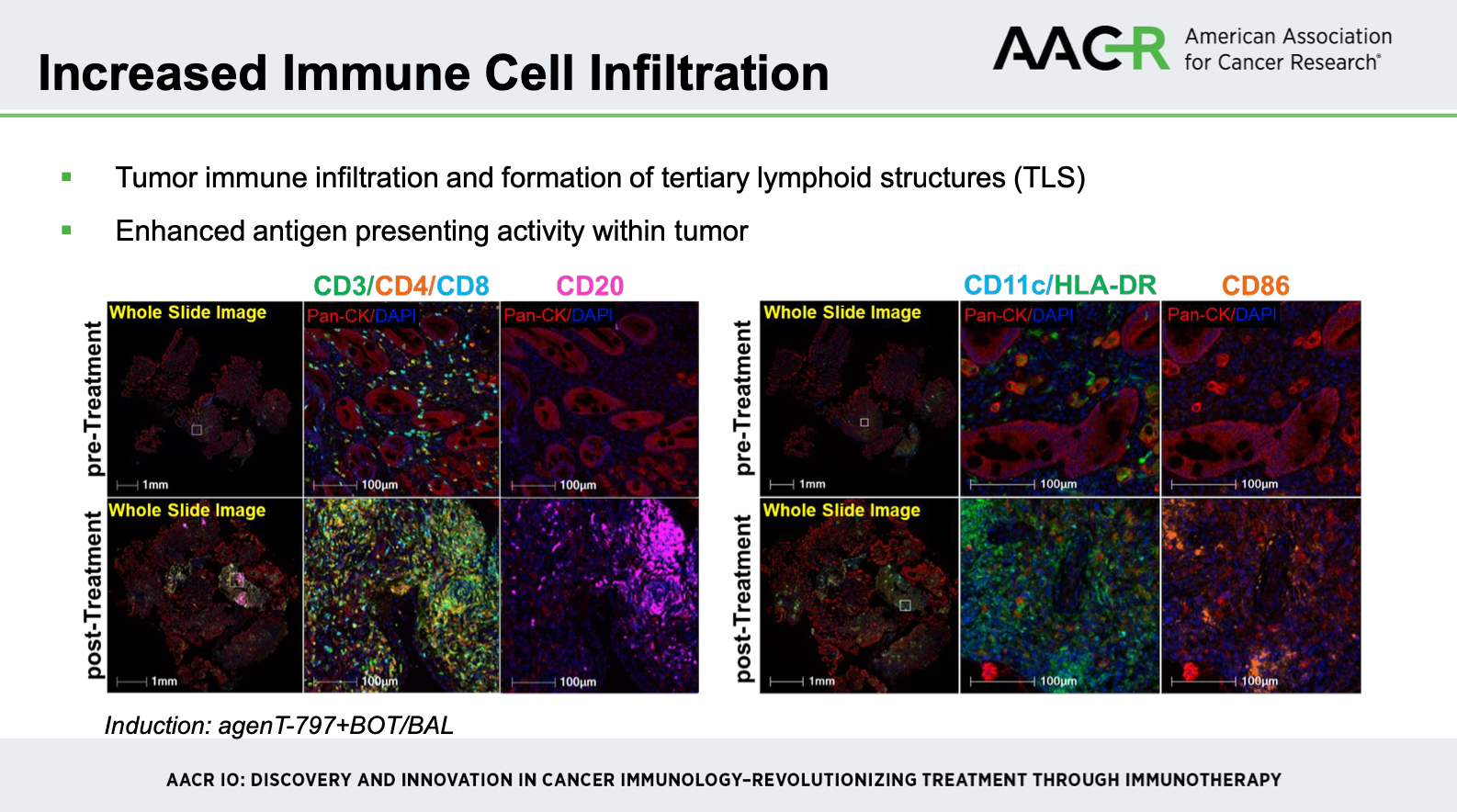
In a Phase II trial at MSKCC (NCT06251973), presented at AACR 2025 by Dr Cytrin and colleagues, patients with advanced PD-1 refractory gastroesophageal adenocarcinoma received AgenT-797 (iNKT cells) in combination with botensilimab, balstilimab, ramucirumab, and paclitaxel. Immune profiling showed increased intratumoral infiltration of CD8+ T cells and antigen-presenting cells, formation of tertiary lymphoid structures, and activation of peripheral central and effector memory T cells. Peripheral biomarkers revealed sustained elevations of IFN-γ, TNF-α, IL-15, and granzyme B, with the most pronounced effects seen in patients receiving the full iNKT + BOT/BAL combination.
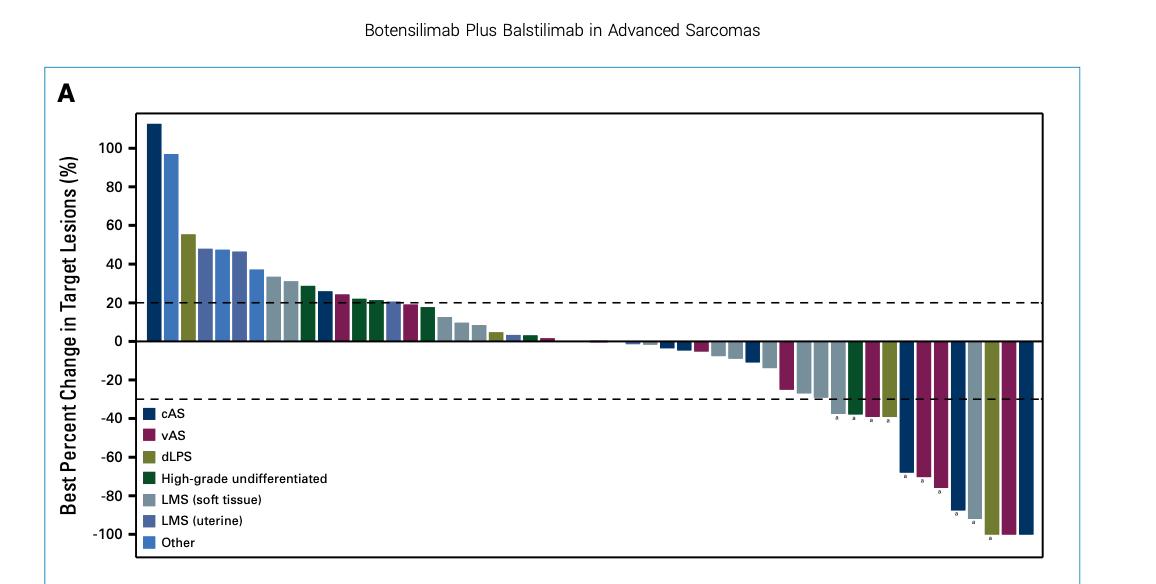
Phase I Study of Bot/Bal in advanced sarcomas
Published in the Journal of Clinical Oncology, a Phase I study of the combination of botensilimab (BOT) and balstilimab (BAL) in relapsed/refractory metastatic sarcomas, a disease setting historically resistant to checkpoint inhibitors. Demonstrates the potential of this dual-agent immunotherapy to drive durable responses across multiple sarcoma subtypes, including angiosarcoma and leiomyosarcoma. The data support ongoing development and further validation of this regimen as a promising option in hard-to-treat tumors.
Key Results
- Overall response rate (ORR): 19.2% across sarcoma subtypes
- Disease control rate (DCR): 65.4%
- Median duration of response (DOR): 21.7 months
- 12-month overall survival (OS) rate: 69%
- Notable activity in angiosarcoma: ORR 27.8%, DCR 77.8%
- Manageable safety profile: No grade 4–5 treatment-related adverse events
These findings underscore Agenus’s growing role in developing next-generation immunotherapies aimed at overcoming resistance mechanisms in solid tumors.
You can read the Article Summary on Oncodaily
FDA’s Evolving Landscape—Can Policy Shifts Accelerate BOT/BAL’s Path to Approval?
The FDA is undergoing a quiet transformation. Under new leadership, the agency is doubling down on expedited pathways, clinical meaningfulness over statistical rigidity, and a greater tolerance for risk in high-need settings. These shifts are particularly relevant to immuno-oncology, where drugs like botensilimab (BOT) and balstilimab (BAL) are beginning to demonstrate real-world impact in patient populations historically resistant to immunotherapy.
For Agenus, the alignment is timely and potentially game-changing. Microsatellite-stable (MSS) colorectal cancer, a disease that comprises the vast majority of CRC cases, has long eluded checkpoint-based strategies. BOT/BAL, however, has delivered response rates in the 17–22% range in heavily pretreated MSS CRC patients, where standard immunotherapies have repeatedly failed. The FDA’s renewed commitment to breakthrough designations and pathway flexibility could enable Agenus to pursue an accelerated approval route, particularly if upcoming trials confirm clinical durability and survival benefit.
Moreover, BOT/BAL’s emerging role in the neoadjuvant setting—where early intervention could prevent surgery or reduce chemotherapy burden—adds strategic momentum. If confirmed in larger trials, this would not only expand the therapeutic window but also offer regulatory optionality in both early and late-line disease.
Agenus’s Financial Landscape: Poised for Growth?
Agenus entered 2025 with improved financial discipline, reducing its Q1 net loss to $26.4 million—a 60% improvement from the same period in 2024—and lowering cash burn to $25.6 million. Operating expenses will fall below $50 million annually by midyear. Although cash reserves declined from $40.4 million to $18.5 million as of 31 March, the company secured a significant strategic partnership with Zydus Lifesciences to strengthen its balance sheet.
Under the agreement, Agenus will receive $75 million in upfront consideration for transferring two U.S.-based biologics manufacturing facilities, plus up to $50 million in contingent payments tied to future production. Additionally, Zydus is investing $16 million in Agenus by purchasing 2.1 million shares at $7.50 per share. The partnership also includes an exclusive license for BOT/BAL in India and Sri Lanka, with Agenus receiving a 5% royalty on regional sales.
First-quarter revenue came in at $24.1 million, driven mainly by non-cash royalties. Between March 31 and its peak on June 6, Agenus stock surged approximately 3.8 times, marking a sharp upward trajectory driven by renewed leadership, strategic partnerships, and growing momentum behind its immunotherapy pipeline.
Botensilimab Among 2024’s Most Promising Cancer Drugs
In 2024, OncoDaily recognized botensilimab as one of the “10 Most Promising Cancer Drugs Not Yet Approved,” citing its broad activity across solid tumors, including microsatellite-stable colorectal cancer, sarcomas, and gastroesophageal cancers. Since then, several drugs on the same list—such as datopotamab deruxtecan, zenocutuzumab, and zolbetuximab—have moved forward with regulatory approvals in early 2025.

Multiple trials have shown high response rates and durable control in heavily pretreated, immune-resistant tumors, and botensilimab now appears poised to follow suit. Its investigational status may soon change as pivotal data mature and the regulatory climate increasingly supports innovation in immunotherapy.
Will Botensilimab be the Breakthrough Agenus Needs?
As oncology advances, so too do the questions.
Can BOT/BAL redefine what’s possible for cancers long thought immune to immunotherapy?
Can Agenus, with Dr. Goldberg now leading its clinical direction, finally pivot from potential to proof?
The answers may hinge as much on timing as on science. Under FDA Commissioner Dr. Marty Makary, the regulatory environment is more receptive— one increasingly open to accelerated pathways for treatments with real promise—and the road to approval may be shorter than before. That shift, combined with credible clinical data and financial recalibration, is breathing new life into the Agenus narrative.
Read The Full Article on CancerWorld
A 23% stock surge after Q1 results signals renewed investor confidence. But beyond market reactions lies something more profound: a rare convergence of data, leadership, and policy that could propel a long-struggling biotech into a defining chapter.
The oncology world isn’t just watching anymore—it’s wondering: What if this time, it really works?
Zydus brings more than manufacturing – they bring market access. With exclusive rights in India and Sri Lanka, Zydus will use its infrastructure to fast-track BOT/BAL development in emerging markets, expanding access and data generation outside the U.S. Simultaneously, U.S. regulatory momentum under FDA Commissioner Dr. Marty Makary is working in Agenus’s favor. New pathways focused on real-world outcomes, flexibility in trial design, and immunotherapy prioritization align with BOT/BAL’s profile, particularly in MSS CRC, where no other checkpoint strategy has succeeded.
With growing clinical evidence, a strengthened financial position, and a new global partnership in place, Agenus is positioned to make meaningful advances in treating cancers that have long resisted immunotherapy. The next phase will be critical—but if current momentum holds, BOT/BAL could mark a significant step forward in oncology.
Written by Amalya Sargsyan, MD, MSc
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
