Zanidatamab, a novel dual HER2-targeted bispecific antibody, has shown promising preclinical and clinical activity in the treatment of HER2-positive cancers, including gastro-oesophageal adenocarcinoma (GEA). This innovative drug simultaneously targets two distinct domains of the HER2 receptor, driving potent anti-tumor activity through mechanisms like HER2 internalization, inhibition of dimerization, and immune-mediated effects such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
In patients with HER2-positive advanced GEA, zanidatamab, when combined with chemotherapy, has demonstrated significant clinical potential in terms of tumor response and survival outcomes. This study explores the efficacy and safety of zanidatamab combined with standard chemotherapy regimens as a first-line treatment for HER2-positive advanced gastro-oesophageal adenocarcinoma, a cancer with generally poor prognosis and limited treatment options.
Title: Zanidatamab plus chemotherapy as first-line treatment for patients with HER2-positive advanced gastro-oesophageal adenocarcinoma: primary results of a multicentre, single-arm, phase 2 study
Published in Lancet Oncology, July 2025
Background
Advanced gastro-oesophageal adenocarcinoma (GEA) remains one of the most challenging cancers to treat, with poor prognosis for most patients. Approximately 20% of patients with GEA have tumors that overexpress the human epidermal growth factor receptor 2 (HER2), making them eligible for HER2-targeted therapies.
Trastuzumab, a HER2-targeted monoclonal antibody, combined with chemotherapy, has been the standard first-line treatment for HER2-positive advanced GEA. However, alternative therapies that may offer improved outcomes are urgently needed. Zanidatamab, a dual-targeted bispecific antibody, has shown promising preclinical and clinical activity against HER2-expressing tumors. This study investigates the use of zanidatamab plus chemotherapy as a first-line treatment for patients with HER2-positive advanced gastro-oesophageal adenocarcinoma.
Methods and Study Design
This phase 2, multicenter, open-label, single-arm trial aimed to evaluate the safety and efficacy of zanidatamab combined with chemotherapy in patients with HER2-positive advanced gastro-oesophageal adenocarcinoma. Patients from Canada, South Korea, and the USA were enrolled between August 29, 2019, and February 18, 2022.
The study was conducted in two parts: part 1 focused on dose-finding and safety, while part 2 evaluated antitumor activity and efficacy. Patients received zanidatamab in combination with three standard chemotherapy regimens: CAPOX (capecitabine plus oxaliplatin), FP (5-fluorouracil [5-FU] plus cisplatin), or modified FOLFOX6 (mFOLFOX6; leucovorin, 5-FU, and oxaliplatin). The study enrolled a total of 46 patients, with 39 male (85%) and 7 female (15%) participants, and a median follow-up of 47.9 months.
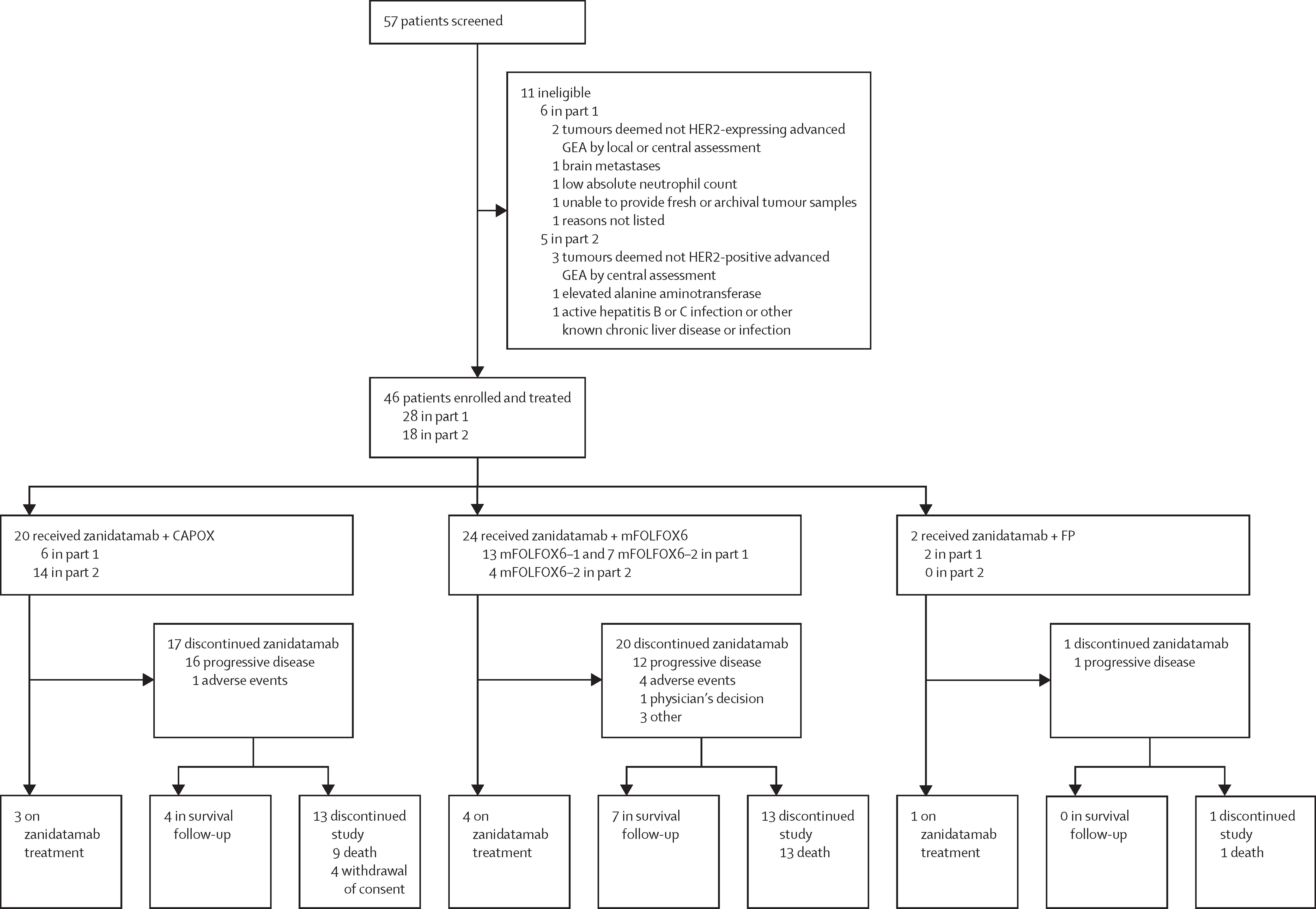
Eligible patients were aged 18 years or older, with untreated, metastatic, or advanced HER2-positive GEA. The study included patients with HER2 IHC 3+ or 2+ (by local or central assessment) and those with confirmed FISH-positive HER2 IHC 2+ in part 2. Patients with uncontrolled cardiovascular, renal, or hepatic diseases, or those with active infections, were excluded. Only patients who had not received prior HER2-targeted therapy or chemotherapy were included.
Results
The study enrolled 46 patients, with a median age of 58 years (range 55–63). Of these, 39 (85%) were male, and 28 (61%) were White, while 17 (37%) were Asian.
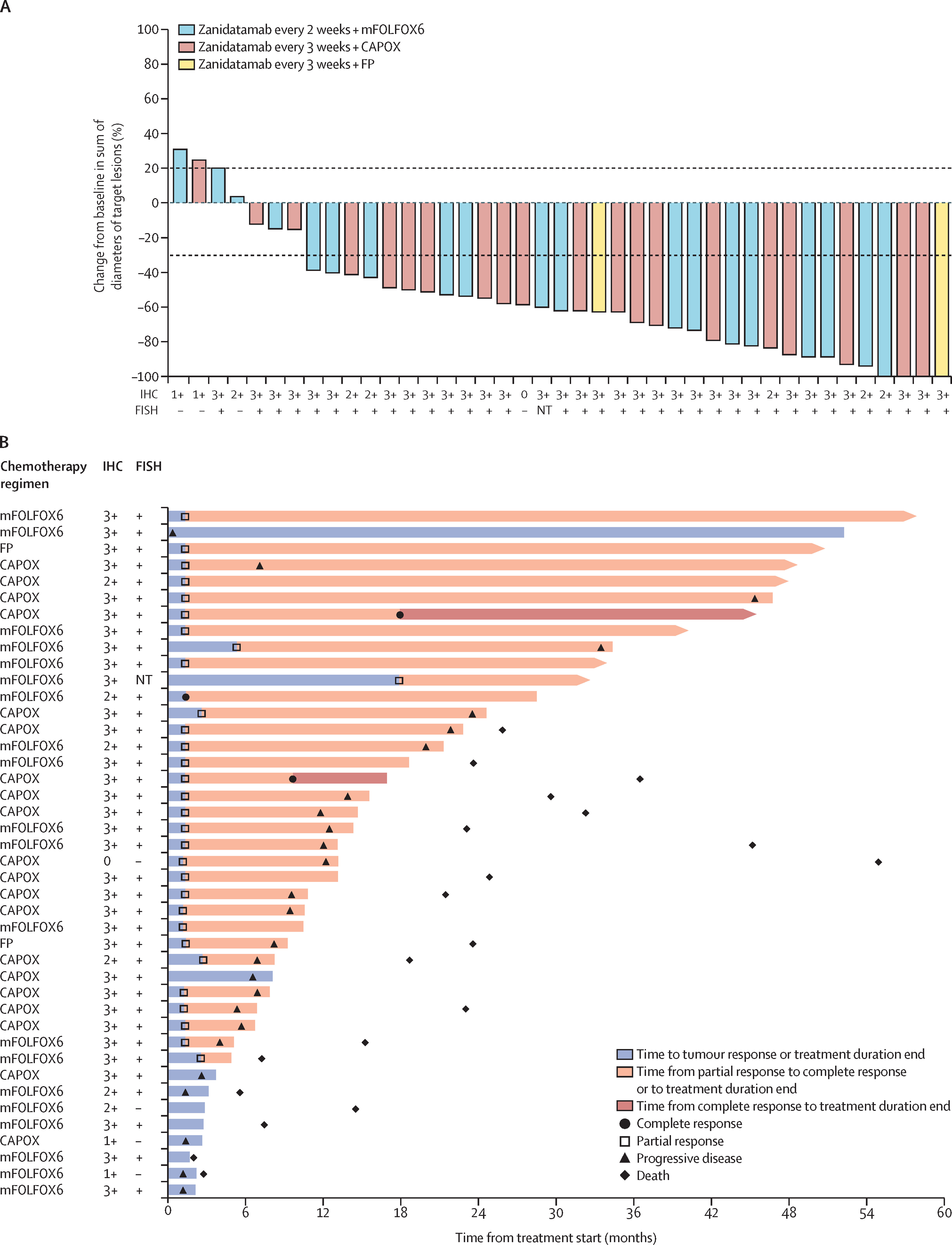
- The confirmed objective response rate (ORR) was 76.2% (95% CI 60.5–87.9) in the overall population, with a median duration of response (DOR) of 18.7 months (95% CI 10.4–44.1).
- The disease control rate (DCR) was 88.1% (95% CI 74.4–96.0), and the clinical benefit rate (CBR) was 78.6% (95% CI 63.2–89.7).
- The median progression-free survival (PFS) was 12.5 months (95% CI 8.2–21.8), while the median overall survival (OS) was 36.5 months (95% CI 23.6-not estimable).
- Among patients with centrally confirmed HER2-positive tumors, the ORR was higher at 84% (31 of 37 response-evaluable patients). Median PFS for this subgroup was 15.2 months (95% CI 9.5–33.4), with a median OS of 36.5 months (95% CI 23.6-not estimable).
- Safety was evaluated across all patients, with the most common treatment-related grade 3 or 4 adverse events being diarrhoea (39%) and hypokalaemia (22%). The incidence of grade 3 diarrhoea decreased after implementing mandatory antidiarrhoeal prophylaxis.
Key Takeaway Messages
- High Response Rates: Zanidatamab plus chemotherapy demonstrated an impressive 76.2% objective response rate, which is higher than the 47% response rate seen in the landmark ToGA trial with trastuzumab and chemotherapy.
- Durability of Responses: The median duration of response was 18.7 months, highlighting the potential for long-term disease control. Notably, 59% of patients had a response lasting over 24 months.
- Manageable Safety Profile: The treatment combination was generally well tolerated, with diarrhoea being the most common adverse event. However, the implementation of mandatory antidiarrhoeal prophylaxis significantly mitigated this issue, improving treatment continuity.
- Efficacy in Centrally Confirmed HER2-Positive Tumors: The ORR was even higher in patients with centrally confirmed HER2-positive tumors (84%), supporting the benefit of precise HER2 testing for patient selection.
- Prolonged Survival Outcomes: Both progression-free survival and overall survival were significantly prolonged, with median OS reaching 36.5 months. These results compare favorably with the current standard of care.
Conclusion
This phase 2 trial demonstrates that zanidatamab, a dual HER2-targeted bispecific antibody, when combined with standard chemotherapy regimens, offers a promising first-line treatment option for patients with HER2-positive advanced gastro-oesophageal adenocarcinoma. The combination showed clinically meaningful and durable antitumor activity, with an impressive objective response rate of 76.2%, and a median overall survival of 36.5 months.
The safety profile was manageable, with proactive management of treatment-related adverse events, particularly diarrhoea. These results are highly encouraging and warrant further investigation, especially in larger, randomized phase 3 trials. Zanidatamab plus chemotherapy represents a potential new standard of care, especially for patients with HER2-positive tumors that are confirmed through central laboratory testing. The ongoing phase 3 trials, including HERIZON-GEA-01, may provide additional insights into the potential role of zanidatamab in the treatment landscape of advanced GEA, especially when combined with immune checkpoint inhibitors like tislelizumab.
This study provides robust evidence for the continued clinical development of zanidatamab in HER2-positive advanced gastro-oesophageal adenocarcinoma and reinforces the importance of precise HER2 testing to ensure optimal patient outcomes.
You can read the full article here.
Written by Sona Karamyan, MD