TWINPEAK study: Spevatamig + gemcitabine + nab-paclitaxel shows efficacy as first-line (1L) therapy in patients with CLDN18.2 positive metastatic pancreatic ductal adenocarcinoma (PDAC).
Key Findings
- Spevatamig (anti-CLDN18.2/CD47 bsAb) with its unique molecular design, mitigates hematological toxicity, and improves gastrointestinal (GI) tolerability (nausea and vomiting) as evidenced by the TWINPEAK study, which includes greater than 100 patients in the United States
- Spevatamig + chemotherapy shows efficacy as first-line (1L) therapy in patients with CLDN18.2 positive metastatic pancreatic ductal adenocarcinoma (mPDAC)
- Spevatamig is a novel immunotherapy which has the potential to become the first innate immunity enhancer (I2E) for a solid tumor indication and is combinable with various other cancer therapies
Disease Background
Metastatic pancreatic ductal adenocarcinoma (mPDAC) is a devastating cancer with a five-year survival rate below 5% (American Cancer Society). Treatment has been largely limited to systemic chemotherapy which only offers a short duration of benefit due to cancer resistance development and cumulative toxicity. The impact on society is only expected to increase, as incidence of PDAC is rising. Thus, novel approaches capable of producing more durable responses are urgently needed.1 Although immunotherapies such as agents targeting PD-1/PD-L1 or CTLA-4 have been explored in many PDAC trials, few have demonstrated meaningful therapeutic benefit.2 Further, due to high rates of serious adverse effects and dose-limiting toxicities, to date, no immunotherapy or biologic has been approved in the 1L treatment setting for patients with mPDAC.
The TWINPEAK study explored whether spevatamig, an IgG1-based bispecific antibody (bsAb) targeting claudin 18.2 (CLDN18.2) and CD47, with an optimized anti-CD47 arm, could improve outcomes in patients with mPDAC.
Target Background
Both CLDN18.2 and CD47 are overexpressed on tumor cells of patients with mPDAC, making them promising therapeutic targets for this disease. CLDN18.2 is also expressed on gastric mucosa, which is believed to cause nausea and vomiting (N/V) when bound by CLDN18.2-targeting agents. Anti-CD47 agents have shown robust efficacy and clinical promise but have been limited by hematological adverse events (AEs). These AEs are believed to be driven by the expression of CD47 on red blood cells (RBCs), platelets and neutrophils, which are rapidly cleared by monocytes/macrophages activated by CD47-targeting agents.
The Molecular Design of Spevatamig
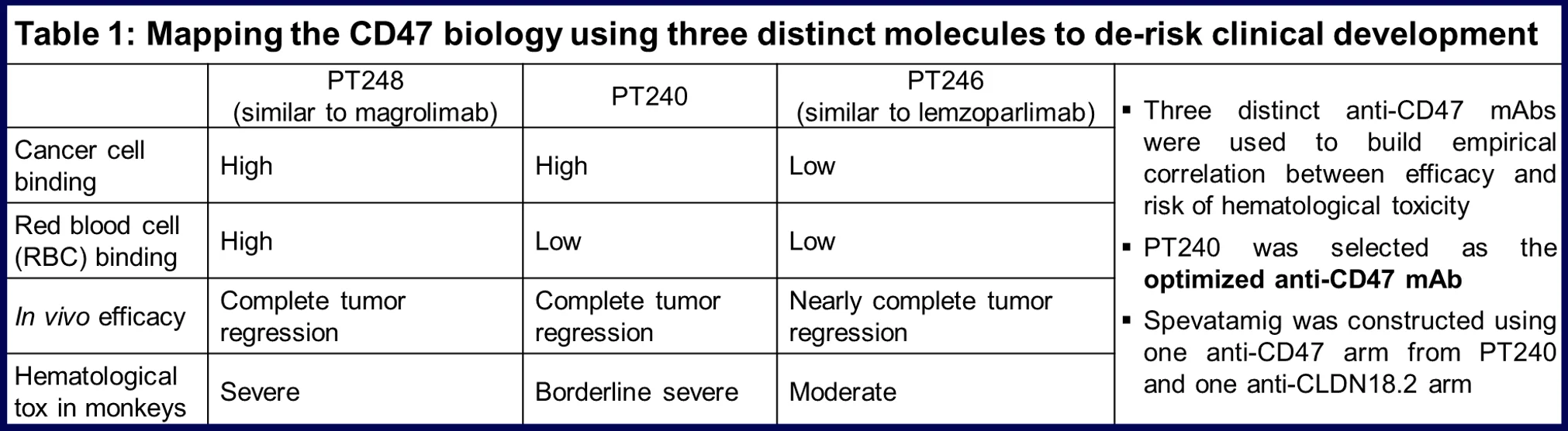
Targeting CLDN18.2 and CD47 as anti-cancer treatment requires a strategy that can mitigate or resolve the GI toxicity associated with CLDN18.2 and the hematological toxicity associated with CD47. Spevatamig was designed with a two-step approach to achieve this goal: 1. Discovery of an optimized anti-CD47 monoclonal antibody (mAb) with preferential binding to cancer cells relative to RBCs; 2. Construction of a native IgG1-like bsAb with one arm from the optimized anti-CD47 mAb (Table 1 and Figure 1).
As the first step, the biology of CD47 was mapped with three distinct anti-CD47 mAbs with varying profiles, leading to the identification of an optimized anti-CD47 mAb, PT240 (Table 1).
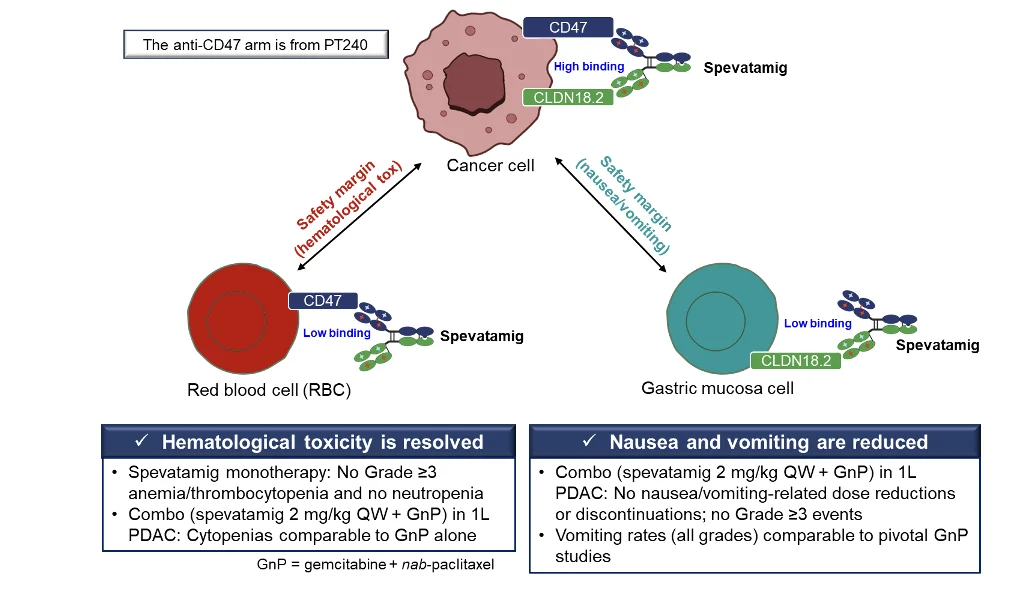
As the second step, spevatamig was constructed using one anti-CD47 arm from PT240 and one anti-CLDN18.2 arm on an IgG1 backbone using PACbody® and SPECpair® (Figure 1). The clinical observations on relevant safety and tolerability from more than 100 patients are summarized below (Figure 1).
Figure 1. Spevatamig’s optimized design mitigates hematological and GI toxicity to improve tolerability.
How does Spevatamig Work?
Unlike many CLDN18.2-targeting agents that usually have two anti-CLDN18.2 arms, spevatamig has only one anti-CLDN18.2 arm that allows it to have weaker binding to gastric mucosa, which does not express CD47, potentially causing less N/V (Figure 1). Thus, spevatamig’s high binding to cancer cells through both arms enables the potential for efficacy while limiting on-target, off-tumor GI toxicity. Likewise, spevatamig has only one anti-CD47 arm that allows it to have weaker binding to RBCs, which do not express CLDN18.2. More importantly, its anti-CD47 arm is from an optimized anti-CD47 mAb that demonstrates higher binding to CD47 on cancer cells than on human RBCs, potentially further reducing hematological AEs (Table 1 and Figure 1).
Spevatamig has been studied in more than 100 patients collectively in monotherapy and combination therapy settings in the US. The clinical proof of concept (POC) of the design is supported by the significantly reduced hematological AEs and N/V observed in the study (summarized in Figure 1). The combination of spevatamig with the standard of care (SOC) chemotherapy gemcitabine + nab-paclitaxel (GnP) in patients with mPDAC is well tolerated, with rates of hematological AEs and rates of Grade ≥ 3 N/V not exceeding rates anticipated from GnP alone.
Mechanistically, chemotherapy (e.g., GnP) induces “eat me” signals on cancer cells which further enhance the immune-activating and cancer killing ability of spevatamig.
Study Design
The TWINPEAK study (NCT05482893) is an ongoing multi-cohort Phase 1 monotherapy dose escalation, and Phase 2 combination expansion and dose optimization study in patients with GI carcinomas. Combination expansion cohorts include various combinations with chemotherapy and/or an immune-checkpoint inhibitor. As of December 12, 2025, 107 patients have been treated with spevatamig in the US collectively in monotherapy and combination settings. Of these patients, 42 with 1L mPDAC have been treated with spevatamig + GnP across several dosing regimens. Data from the 2 mg/kg weekly (QW) + GnP regimen is presented, with data from dosing regimen > 2 mg/kg QW spevatamig + GnP still maturing.
Treatment Regimen
- 2 mg/kg QW spevatamig + GnP.
- GnP dosing was at the discretion of the treating physician and per Institutional Standard, with the starting regimen being 3 weeks on, one week off.
- GnP modifications were allowed at the discretion of the treating physician.
- Treatment continued until disease progression, intolerable toxicity or withdrawal of consent.
Key Endpoints
- Safety and tolerability
- Objective response rate (ORR)
- Disease Control Rate (DCR)
- Progression-free survival (PFS)
- Overall survival (OS)
Patient Population
- mPDAC patients, who are treatment naïve for their metastatic disease and have no contraindications to receive GnP.
- Patients must possess measurable disease per RECIST 1.1.
- Patients who received any neoadjuvant and/or adjuvant therapy must have completed the therapy at least 12 months prior to study treatment.
- Patients must present with ≥ 10% (≥ 2+ staining) CLDN18.2 positive tumor cells from a biopsy specimen.
Efficacy Overview
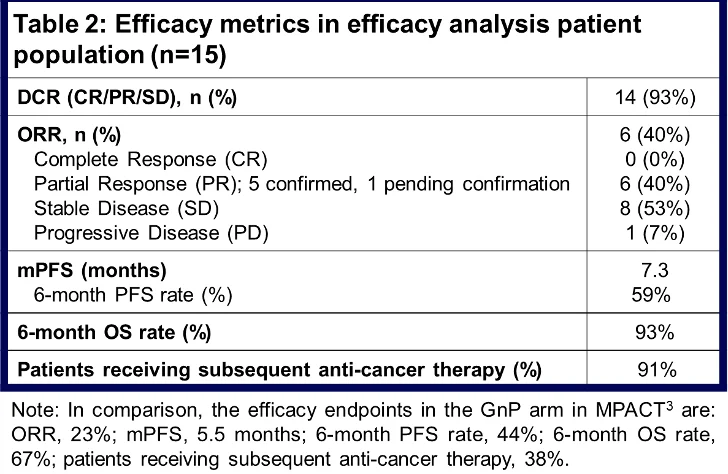
In the 2 mg/kg QW spevatamig + GnP 1L mPDAC dosing regimen (n=15), efficacy data are promising relative to metrics from pivotal studies of GnP in 1L mPDAC.3,4
Patients included in this dosing regimen possess representative disease and baseline characteristics relative to patients tested in pivotal studies of GnP3, 4. The DCR was 93%. The ORR was 40%, with 6 patients achieving partial response, 5 of which have been confirmed and one of which remains pending confirmation. The mPFS was 7.3 months (compared to 5.5 months in MPACT and 5.6 months in NAPOLI-3 3,4) with a 6-month PFS rate of 59%. The mOS was 13.2 months (compared to 8.5 months in MPACT and 9.2 months in NAPOLI-3, 3,4) and still maturing while 6-month OS rate was 93%. Currently, 3 patients remain on the study, and 6 patients are in long-term follow-up; 91% of patients received subsequent anti-cancer therapies upon progression (Table 2).
There is no apparent correlation between ORR and CLDN18.2 score, with responses observed across CLDN18.2 scores ≥ 10% (≥ 2+ staining). Of note, 85% of patients who were screened met this CLDN18.2 threshold requirement. Given the prevalence of CLDN18.2 tumor expression in mPDAC and the lack of correlation observed between expression and response, the requirement for CLDN18.2 tumor expression has been removed for patients currently enrolling into this 1L mPDAC cohort. Patients will still undergo retrospective tumor testing for CLDN18.2 to correlate efficacy across the spectrum of all CLDN18.2 expression.
Safety
A favorable safety profile has been observed in the TWINPEAK study. In monotherapy, no CRS or DLTs were observed. The maximum tolerated dose (MTD) has not been reached in either the monotherapy or combination therapy setting. No Grade ≥ 3 treatment-emergent anemia, neutropenia or thrombocytopenia were observed during the study. One patient experienced Grade 3 anemia following termination of the study, which was deemed not related to spevatamig, and recovered. Nausea and vomiting, however, were observed. Starting from 3 mg/kg QW in monotherapy, an optimized premedication regimen and infusion time adjustment improved patient symptoms.
At 2 mg/kg QW spevatamig + GnP dose level, the rates of anemia, neutropenia and thrombocytopenia were comparable to those observed in the GnP treatment arms from pivotal trials (NAPOLI-3 4). No Grade ≥ 3 treatment-emergent nausea or vomiting events were reported, and no dose reductions or treatment discontinuations due to nausea or vomiting occurred. No CRS was observed.
These data are clinical POC that the molecular design of spevatamig appears to mitigate hematologic toxicity and improve GI tolerability.
Clinical Summary
Overall, spevatamig + GnP is well tolerated, with no significant additive toxicity when compared to GnP alone. The 2 mg/kg spevatamig + GnP dosing regimen demonstrated improved efficacy when compared with pivotal studies of GnP alone in 1L mPDAC, suggesting that the addition of spevatamig may provide additional clinical benefit to patients. Efficacy data from the > 2 mg/kg spevatamig + GnP dosing regimen is maturing and will inform whether higher doses of spevatamig may lead to greater clinical benefit for patients with mPDAC.
Overall Takeaways
Patients with mPDAC have very few effective treatment options beyond chemotherapy. Even in patients who initially benefit from chemotherapy, the duration of benefit is limited. Immunotherapy offers the potential for longer duration of benefit for patients; however, mPDAC represents a cold tumor type to approaches targeting adaptive immunity with targets such as PD-1/PD-L1 and CTLA-4.2 Anti-CD47 agents have shown robust efficacy and clinical promise but have been limited by hematological AEs.
For the first time, clinical POC data were presented to show that CD47 can be targeted with minimal hematological toxicity and significant efficacy. Significant resolution of the hematological AEs associated with CD47 with the unique molecular design of spevatamig is a key milestone in leveraging innate immunity to treat mPDAC. Spevatamig has the potential to become the first innate immunity-targeting drug for a solid tumor indication.
Spevatamig has a low CLDN18.2 score requirement (10%), covering approximately 85% of patients with mPDAC. This threshold is lower than the threshold requirement of many other CLDN18.2 targeting therapies.5
Spevatamig has a favorable safety and tolerability profile when compared with other therapies targeting CLDN18.2 such as antibody-drug conjugates (ADCs), mAbs, and T-cell engagers. It has less severe hematological toxicity and better combinability with SOC treatment backbones relative to ADCs. It does not cause CRS and is administered in an outpatient setting relative to T-cell engagers. It causes less severe nausea and vomiting relative to anti- CLDN18.2 mAbs.
As an immunotherapy, spevatamig’s combinability and differentiated mechanism of action make it a natural partner for backbone SOC chemotherapy in mPDAC. These aspects of the molecule also enhance the prospect of combining it with potential future treatment backbones in this disease such as KRAS inhibitors. By introducing a novel immunotherapy-based approach, spevatamig offers patients with PDAC access to mechanisms of action not previously available in this disease.
The full dataset of spevatamig + GnP in 1L PDAC can be viewed here.
References
- Bussetty A, Shen J, Benias P, et al. Incidence of Pancreas and Colorectal Adenocarcinoma in the US. JAMA Netw Open. 2025;8(4):e254682.
- Principe D, Korc M, Kamath S, Munshi H, et al. Trials and Tribulations of Pancreatic Cancer Immunotherapy. Cancer Lett. 2021; 504: 1–14.
- Van Hoff DD, Ervin T, Arena F, et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N Engl J Med. 2013;369(18):1691-703.
- Wainberg ZA, Melisi D, Macarulla T, et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402(10409):1272-1281.
- https://newsroom.astellas.com/2025-10-13-Astellas-Confirms-Phase-2-GLEAM-Trial-Did-Not-Meet-Primary-Endpoint-of-Overall-Survival-in-Patients-with-Metastatic-Pancreatic-Cancer.