TUXEDO-3, a phase 2 clinical trial, evaluated the investigational HER3-directed antibody–drug conjugate patritumab deruxtecan in patients with leptomeningeal metastases (LMD) from solid tumors—a population with critically limited treatment options and extremely poor prognosis. LMD affects approximately 5–10% of patients with advanced cancers such as lung, breast, and melanoma, and is associated with rapid neurological decline and median survival often measured in weeks. Existing therapies offer minimal benefit, especially due to the challenge of delivering effective agents across the blood–brain and blood–CSF barriers.
TUXEDO-3 aimed to address this unmet need by exploring whether patritumab deruxtecan, previously shown to penetrate CNS tissue, could offer clinical benefit for patients with HER3-expressing tumors and leptomeningeal spread.
Title: Patritumab deruxtecan in leptomeningeal metastatic disease of solid tumors: the phase 2 TUXEDO-3 trial
Authors: Matthias Preusser, Javier Garde-Noguera, Juan José García-Mosquera, María Gion, Richard Greil, Miriam Arumi, Manuel Ruiz-Borrego, Antonio Llombart-Cussac, María Valero, Javier Cortés, Marta Campolier, José Antonio Guerrero, Paula González-Alonso, Carlos Jiménez-Cortegana, Jose Rodríguez-Morató, Marta Vaz-Batista, Felicitas Oberndorfer, Maximilian Marhold, Anna Sophie Berghoff, Julia Furtner, Thorsten Fuereder, Rupert Bartsch
Published in Nature, 2025
Background
Leptomeningeal metastatic disease (LMD) arises when solid tumors spread to the leptomeninges or cerebrospinal fluid, affecting up to 10% of patients with advanced solid cancers—most commonly lung, breast, and melanoma—and is associated with severe neurological deficits, poor prognosis, and limited treatment options. Patritumab deruxtecan (HER3‑DXd) is a HER3-targeted antibody–drug conjugate previously active in breast and lung cancers, with potential CNS penetration and relevance to metastases.
Methods and Study Design
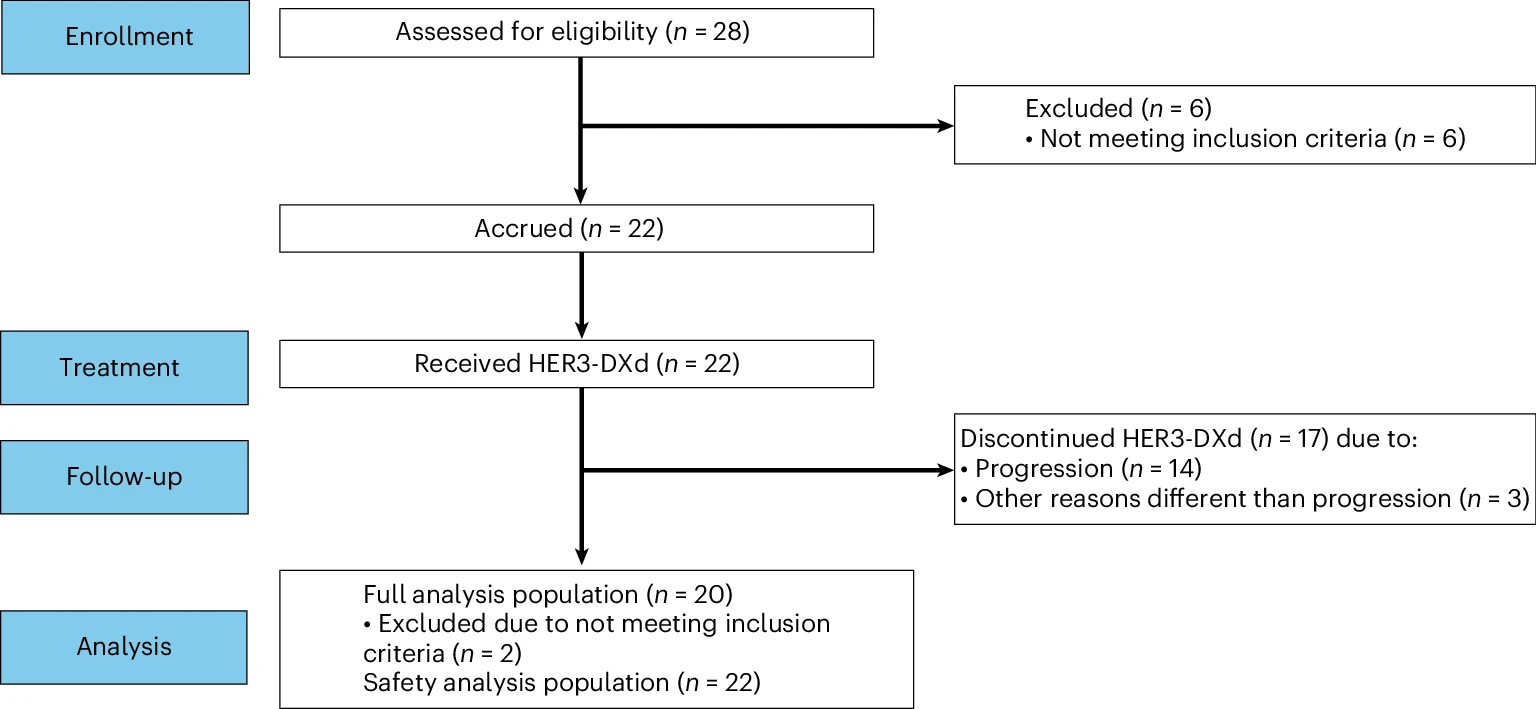
The analysis focused on cohort 3 of the TUXEDO-3 phase 2 trial, evaluating intravenous patritumab deruxtecan in patients with confirmed LMD from solid tumors. The study assessed safety, tolerability, neurological symptom responses, CSF cytology, imaging, and survival outcomes following treatment.
This was a single-arm phase 2 cohort evaluating efficacy and safety of HER3‑DXd in LMD. Patients previously treated for solid tumors with leptomeningeal involvement were enrolled. Key endpoints included neurological symptom improvement, cytological clearance in cerebrospinal fluid, radiographic stabilization or response, safety profile, and survival metrics .
Results
The results from TUXEDO-3 demonstrated encouraging clinical activity of patritumab deruxtecan in patients with leptomeningeal metastases, showing improvements across neurological symptoms, cerebrospinal fluid cytology, and radiographic assessments, with a manageable safety profile.
- Neurological improvements: A meaningful proportion of LMD‐affected patients experienced alleviation of debilitating symptoms such as headaches, radicular pain, gait disturbances, cranial nerve palsies, and nausea.
- Cytological clearance: Among evaluable patients, several achieved negative CSF cytology after treatment, indicating reduction or clearance of malignant cells.
- Radiographic response/stabilization: Imaging of leptomeningeal disease showed stabilization or partial responses in multiple cases.
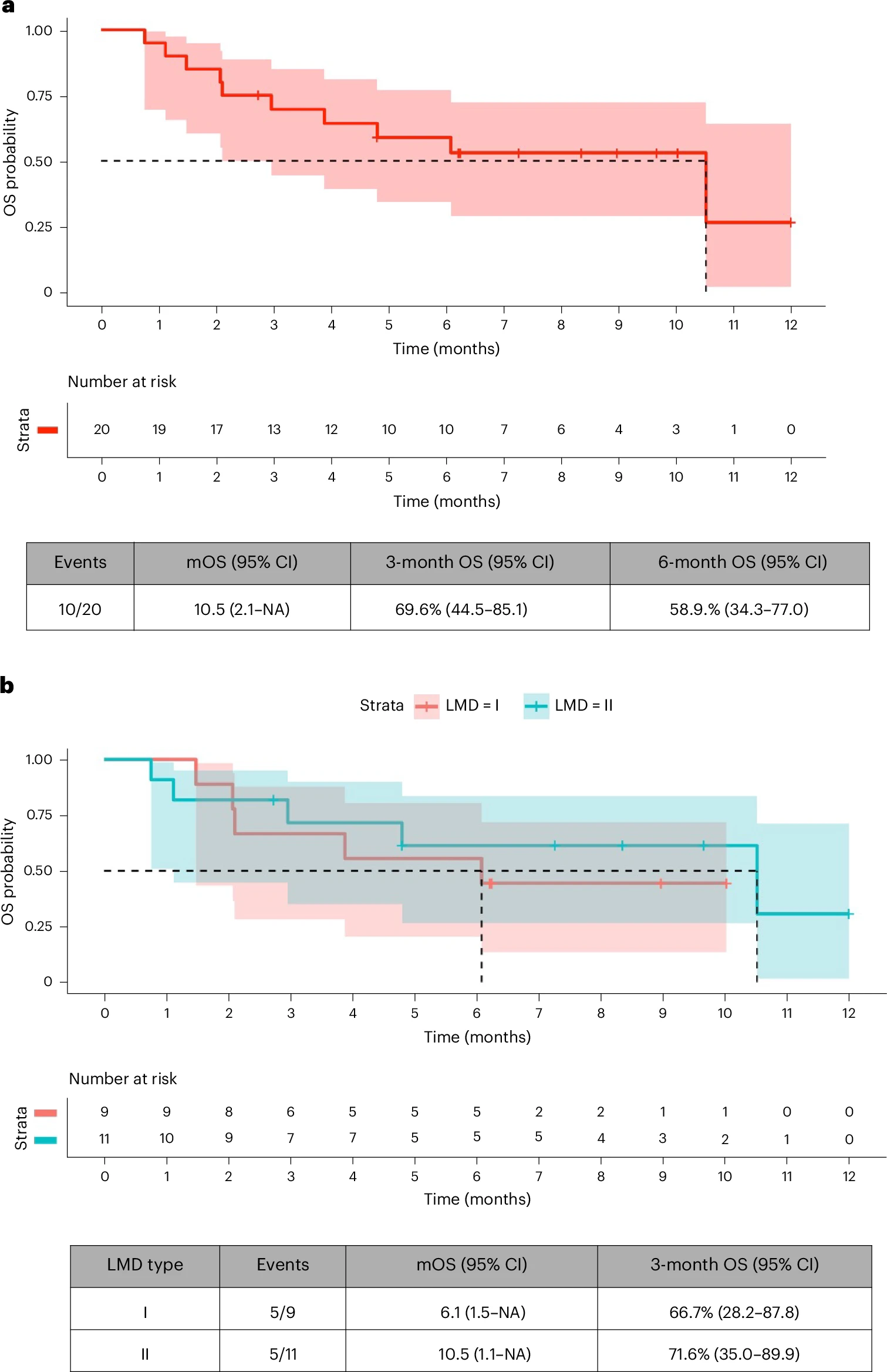
- Survival: While median overall survival was not explicitly detailed in press summaries, investigators reported clinically relevant efficacy in a cohort that historically has survival measured in weeks to a few months.
- Safety: The agent was well tolerated, with an acceptable safety profile. No unexpected CNS toxicities were noted; the adverse events aligned with known effects of HER3‑DXd in other tumor types.
Key Findings
- HER3‑DXd showed meaningful clinical activity in patients with leptomeningeal metastases from solid tumors, a population with historically dismal outcomes.
- Improvement in neurological symptoms and CSF cytology clearance suggest on-target effectiveness in CNS compartments.
- The treatment exhibits radiographic stability or partial response, highlighting potential disease control.
- The safety profile remained acceptable, with no new CNS-related toxicity signals. This is particularly notable given the fragile status of LMD patients.
Key Takeaway Messages
- Patritumab deruxtecan represents a promising new therapeutic option for LMD secondary to HER3‑expressing solid tumors, addressing an urgent unmet need.
- Use of targeted antibody‑drug conjugates can achieve clinical and cytological responses in the CNS, an area notoriously refractory to systemic therapy.
- Symptom relief and disease stabilization in LMD can significantly affect quality of life, even if survival remains limited.
- The tolerability of HER3‑DXd encourages further investigation and potential expansion into larger clinical studies or combination regimens.
Conclusion
In patients with leptomeningeal metastatic disease from solid tumors, patritumab deruxtecan demonstrated encouraging efficacy—ranging from neurological symptom improvement and CSF cytology clearance to radiographic disease control—while maintaining an acceptable safety profile. Given that LMD typically portends survival measured in weeks, these results mark a potential paradigm shift. HER3‑targeted ADC therapy warrants further confirmation, but could notably extend and improve quality of life for patients facing this devastating complication.
You can read the full article here.
Written by Sona Karamyan, MD.


