Early Recurrence in Triple-Negative Breast Cancer After Neoadjuvant Immunotherapy
Despite recent advances in triple-negative breast cancer (TNBC), early recurrence remains a formidable challenge—even in the era of immunotherapy. A new pooled analysis of five major neoadjuvant clinical trials underscores the urgent need to better understand and address early relapsing disease, particularly in high-risk patients who fail to achieve a pathological complete response (pCR).
Shifting Standards, Persistent Risk
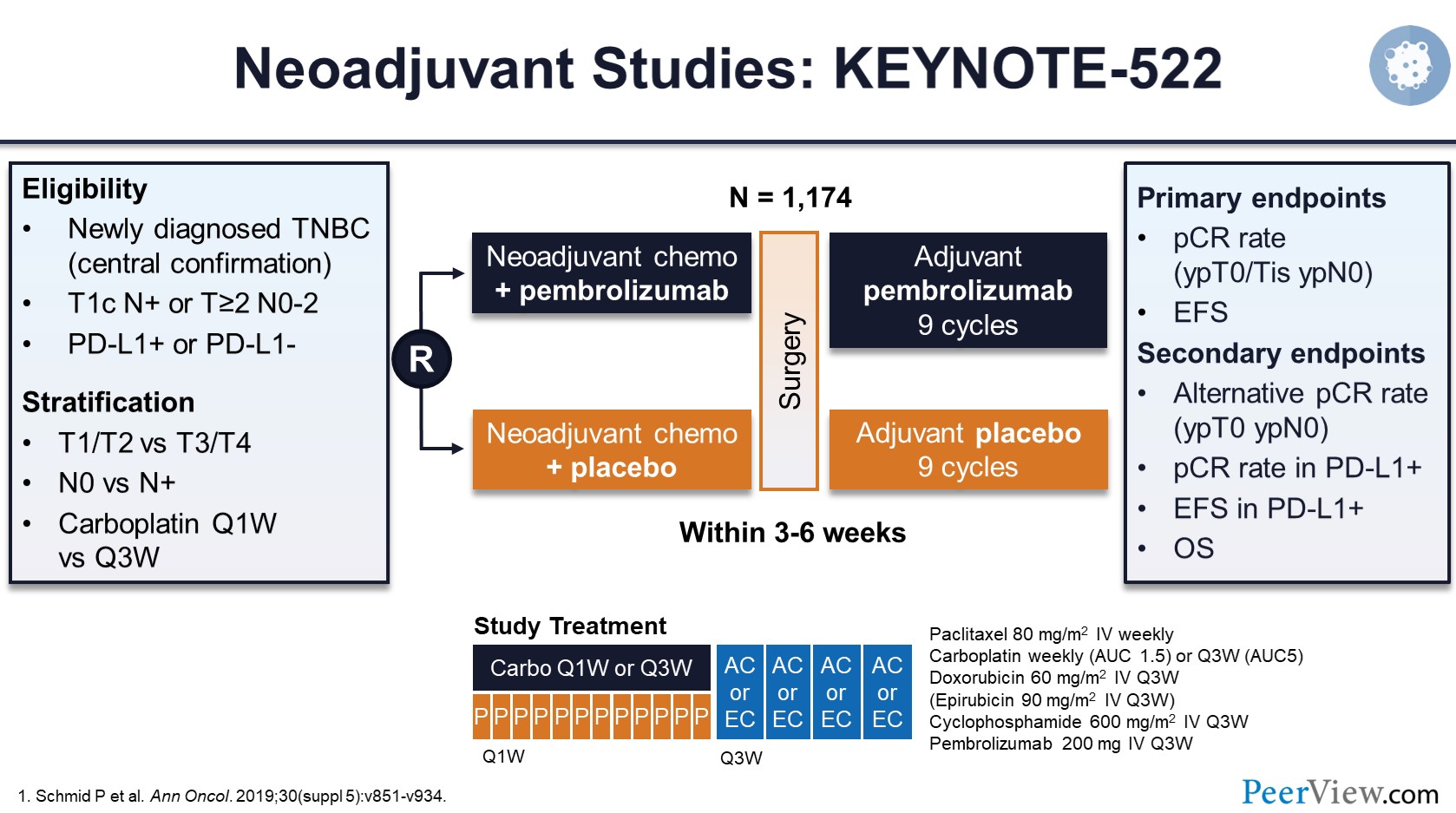
The treatment landscape for stage II/III TNBC has evolved with the integration of immune checkpoint inhibitors (ICIs) into neoadjuvant chemotherapy, driven largely by the KEYNOTE-522 trial and supported by subsequent studies including GeparNuevo, NSABP-B59/GeparDouze, and IMpassion031. While these trials demonstrated improved pCR rates and reductions in recurrence risk, they also revealed a critical insight: most recurrences still occur within 24 months of treatment initiation.
Across the five trials included in this study, early events (≤24 months) accounted for 65% to over 80% of all recurrences. Alarmingly, the majority of these relapses occurred within just 18 months—highlighting a vulnerable period shortly after completing adjuvant therapy.
Recurrence Timing by Response Type in Triple-Negative Breast Cancer
-
Among patients who achieved pCR, the 48-month recurrence rate was low (4.5%–6.8%). However, nearly half of these events still occurred early, within 24 months.
-
In contrast, patients with residual disease experienced far higher event rates (23.7%–38.9%), and early relapsesmade up 70%–89% of their total recurrences.
These patterns confirm that residual disease after neoadjuvant immunotherapy marks a population at exceptional risk, and suggest that pCR may not fully protect against early recurrence.
Biologic Resistance and Limited Options
Early-relapsing TNBC likely represents a biologically distinct subtype with inherent resistance to both chemotherapy and immunotherapy. Yet, this subgroup remains underrepresented or outright excluded in many metastatic TNBC trials.
For instance, the IMpassion132 trial—the only phase III study dedicated to early-relapsing TNBC—did not show a survival benefit for atezolizumab in PD-L1+ patients. Similarly, KEYNOTE-355 yielded inconclusive results in the small subgroup with a disease-free interval (DFI) of 6–12 months. Most new trials (e.g., ASCENT-03, TROPION-Breast05) exclude patients with a DFI <6 months, further limiting treatment options.

Read More About Immunotherapy for Breast Cancer
Implications for Research and Follow-Up
This comprehensive analysis highlights the critical need for trials specifically designed for early-relapsing TNBC, particularly in patients with prior ICI exposure. Promising strategies may include:
-
Combining ICIs with ADC-based or anti-angiogenic therapies
-
Investigating bispecifics and other next-generation immunotherapies
-
Stratifying follow-up based on early risk, potentially incorporating ctDNA monitoring or more intensive imaging in the first two years
Conclusion
While neoadjuvant chemo-immunotherapy has reshaped the treatment of early-stage TNBC, this analysis underscores a sobering truth: early recurrence remains the dominant driver of treatment failure, especially in patients with residual disease.
Identifying, understanding, and targeting these early relapsing tumors should be a top research priority—both for clinical trial design and real-world surveillance strategies.
You Can Watch More on OncoDaily Youtube TV
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
