At the ESMO Congress 2025, Prof. Xichun Hu presented the results of a pivotal phase 3 clinical trial (NCT06968585) evaluating trastuzumab botidotin (A166) in patients with HER2-positive unresectable or metastatic breast cancer (mBC) who had received prior anti-HER2 therapy. The study showed that trastuzumab botidotin significantly improved progression-free survival (PFS) compared with trastuzumab emtansine (T-DM1), establishing it as a potential new therapeutic option in the post-trastuzumab treatment setting.
Background
Despite substantial therapeutic progress in HER2-positive breast cancer, many patients ultimately develop resistance to current anti-HER2 agents. Although drugs such as trastuzumab, pertuzumab, and T-DM1 have improved outcomes, durable disease control remains challenging for those with metastatic or unresectable disease. Therefore, more potent and selective HER2-directed agents are needed to extend survival while maintaining quality of life.
Trastuzumab botidotin (A166) is a next-generation HER2-targeted antibody–drug conjugate (ADC) consisting of a monoclonal antibody linked to the cytotoxic payload Duo-5 through a stable, protease-cleavable valine-citrulline linker. This design enables targeted intracellular delivery of the cytotoxic agent, minimizing systemic toxicity and maximizing tumor-specific killing. In a prior phase 1 study, A166 demonstrated encouraging antitumor activity and manageable safety in heavily pretreated patients with HER2-positive breast cancer, supporting its further evaluation in a phase 3 head-to-head comparison with T-DM1.
Study Design and Methods
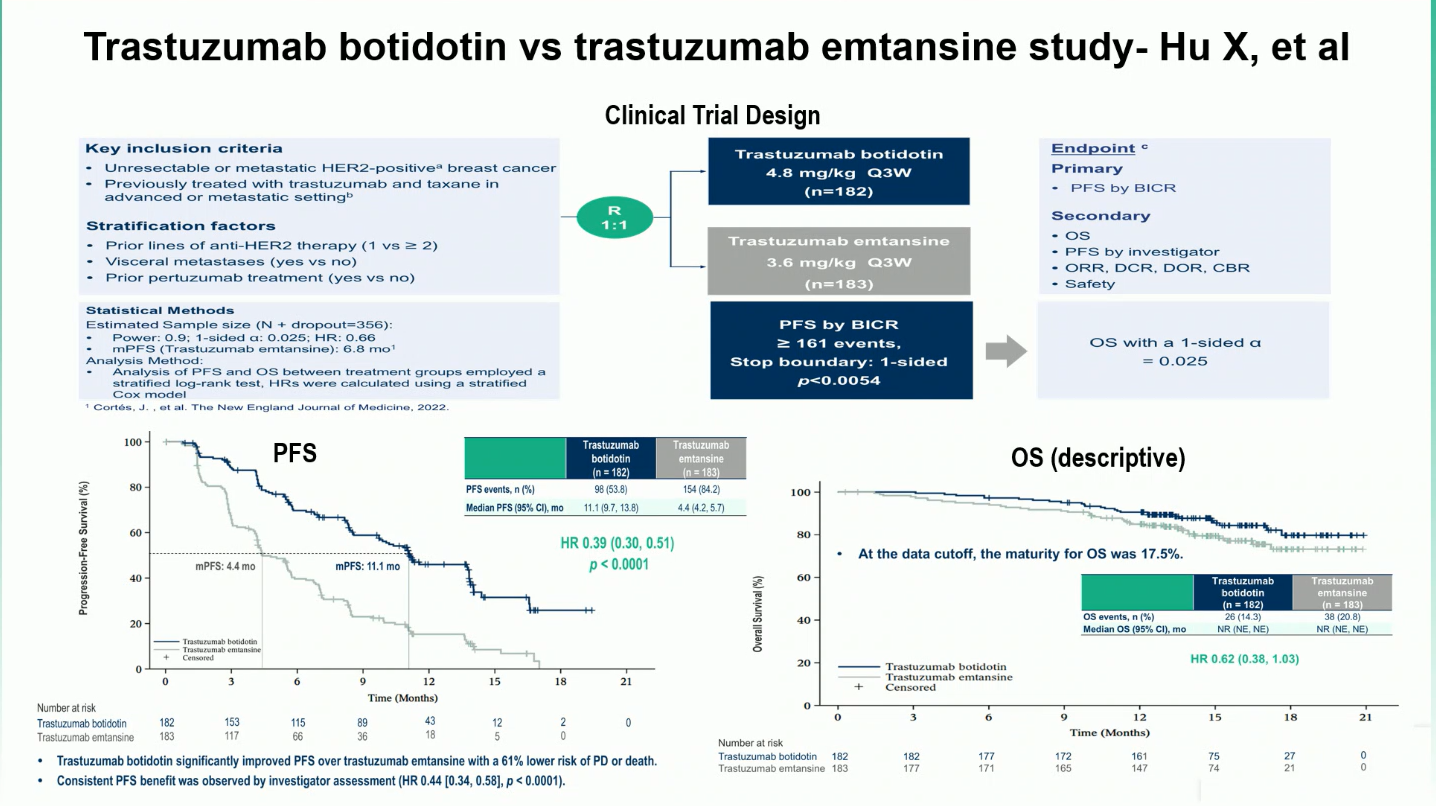
This multicenter, randomized, open-label phase 3 trial enrolled 365 patients with HER2-positive unresectable or metastatic breast cancer who had received at least one prior HER2-directed therapy. Participants were randomized in a 1:1 ratio to receive either A166 (4.8 mg/kg IV every three weeks) or T-DM1 (3.6 mg/kg IV every three weeks) until disease progression or unacceptable toxicity.
The primary endpoint was progression-free survival (PFS) as assessed by blinded independent central review (BICR) per RECIST v1.1. Secondary endpoints included overall survival (OS), objective response rate (ORR), duration of response (DOR), and safety.
Results
The phase 3 trial achieved its primary endpoint, showing a significant efficacy advantage for trastuzumab botidotin (A166) over trastuzumab emtansine (T-DM1). With a median follow-up of nearly 15 months, the data demonstrated notable improvements in progression-free survival, objective response rate, and duration of response.
- Median PFS: 11.1 months with A166 vs 4.4 months with T-DM1 (HR 0.39; 95% CI 0.30–0.51; p < 0.0001).
- The PFS benefit was consistent across subgroups — HR 0.36 for patients with one prior line and HR 0.39 for those with two or more prior lines of HER2-directed therapy.
- Objective response rate (ORR): 76.9% with A166 vs 53.0% with T-DM1.
- Median duration of response (mDOR): 12.2 months with A166 vs 5.7 months with T-DM1.Overall survival (OS): Data remain immature, but an early trend favored A166 (HR 0.62; 95% CI 0.38–1.03).
Overall, A166 demonstrated robust and durable antitumor activity, maintaining clinical benefit across patient subgroups and reinforcing its potential to reshape treatment sequencing in HER2-positive metastatic breast cancer.
Safety Profile
The treatment was generally well tolerated. Grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 69.8% of patients treated with A166 and 63.7% with T-DM1. The most common grade ≥3 A166-related toxicities included ocular disorders such as corneal disorder, dry eye, and blurred vision. These events were largely reversible with dose adjustment and supportive care.
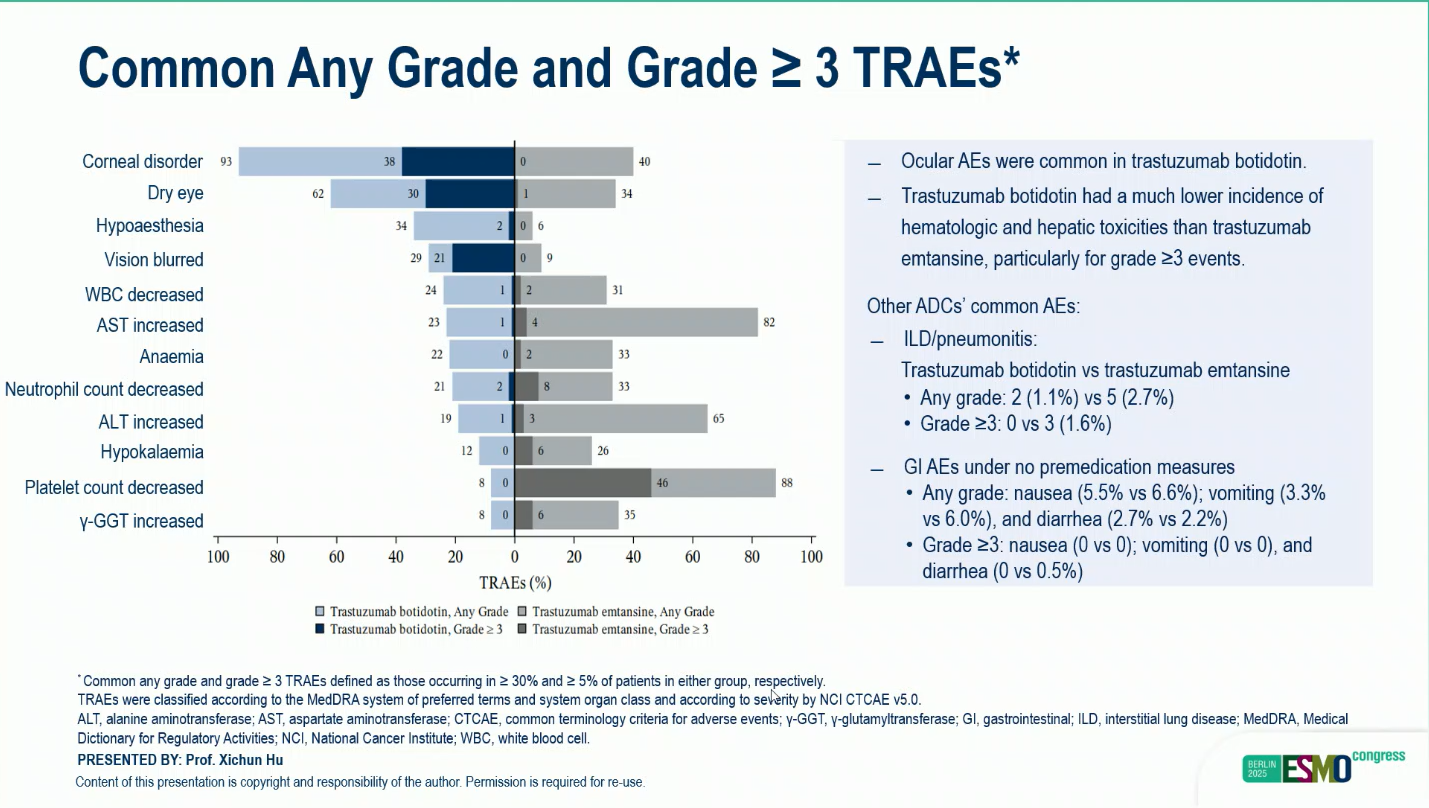
Among A166-treated patients who developed ocular events, 20.3% experienced limitations in instrumental activities of daily living (ADL), and 7.1% reported limitations in self-care ADL. Resolution occurred in 86.5% and 92.3% of patients, respectively. Discontinuation due to TEAEs was infrequent (1.1% with A166 vs 3.8% with T-DM1), and no treatment-related deaths occurred in the A166 arm.
Clinical Implications
The findings presented by Prof. Hu suggest that trastuzumab botidotin (A166) could become a new standard of care for patients with previously treated HER2-positive metastatic breast cancer. Its marked PFS improvement, higher response rates, and favorable tolerability profile—especially when compared with T-DM1—represent a meaningful step forward in HER2-targeted therapy.
While ongoing follow-up will determine the extent of overall survival benefit, the ESMO 2025 presentation underscores the growing role of next-generation ADCs in reshaping the treatment landscape for HER2-positive breast cancer and offers renewed hope for patients with limited therapeutic options.
You can read the full abstract here.


