The Danish phase II TOMBOLA trial evaluated a clinically pragmatic question in muscle-invasive bladder cancer (MIBC): can tumor-informed ctDNA identify who truly needs adjuvant immunotherapy after neoadjuvant chemotherapy (NAC) and radical cystectomy (RC)—and who can safely avoid it?
Rather than assigning treatment solely by pathology and clinical risk features, TOMBOLA used serial, patient-specific ctDNA testing (ddPCR) to guide postoperative escalation and de-escalation decisions, aiming to reduce both undertreatment (missing molecular relapse) and overtreatment (treating patients with no residual disease signal).
TOMBOLA Trial
Why This Matters: Post-Cystectomy Risk Stratification Still Misses the Biology
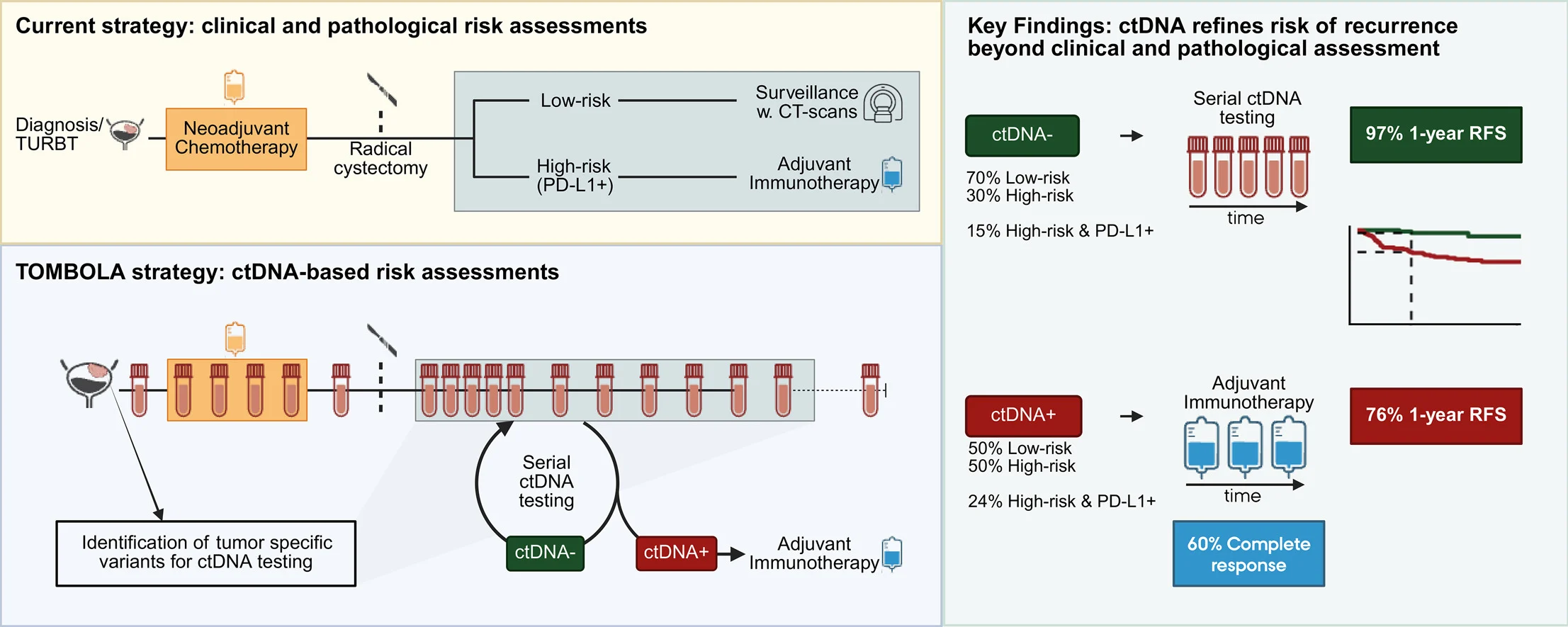
Even after NAC + RC, relapse rates remain high, and conventional staging cannot reliably detect minimal residual disease (MRD) early enough for optimal intervention. Adjuvant immunotherapy is typically restricted to “high-risk” features and sometimes PD-L1 status—yet ctDNA may identify relapse risk beyond these categories, and may also reassure clinicians that some “high-risk” patients are molecularly disease-free.
Study Design and Methods
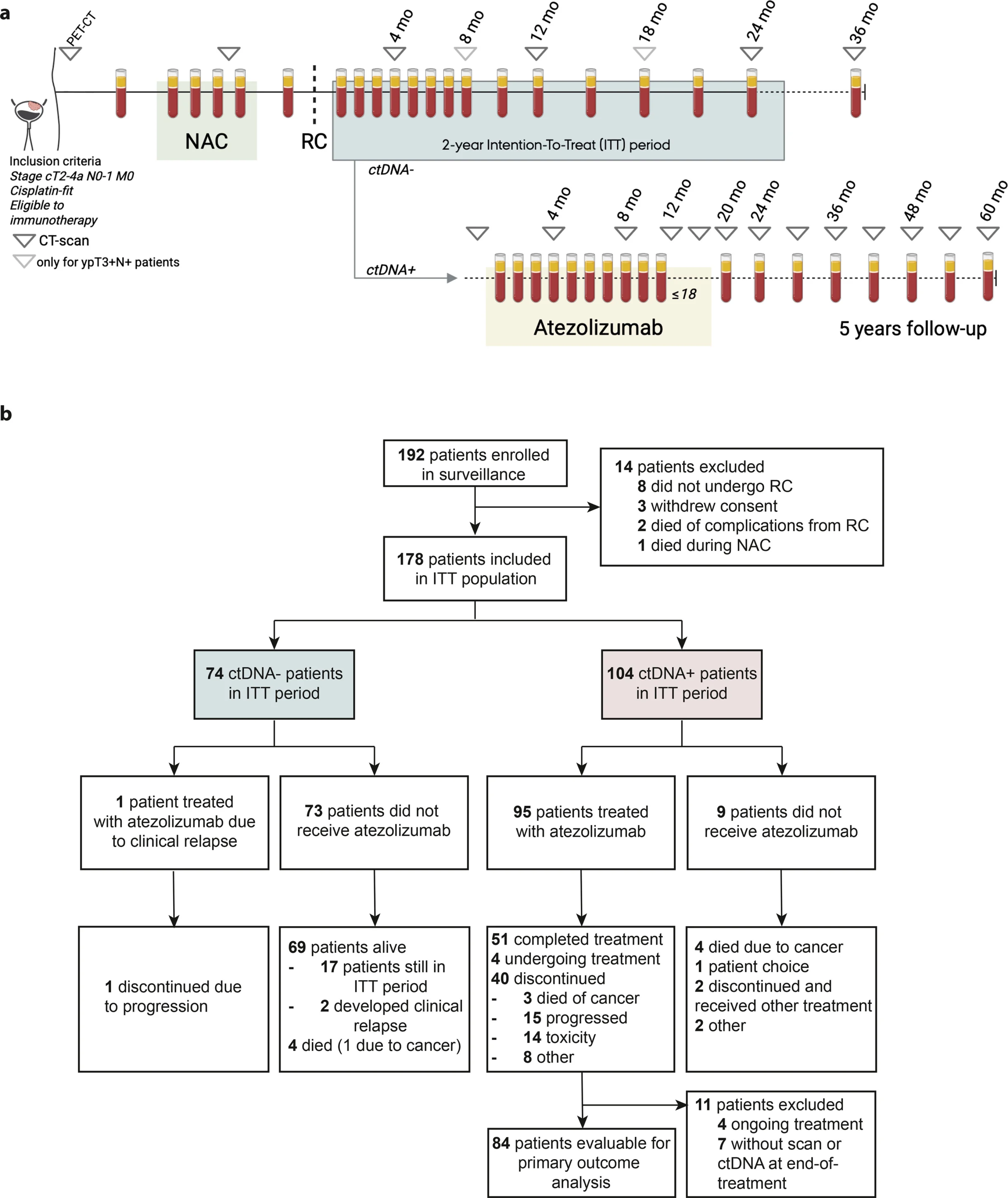
TOMBOLA was a Danish multicenter, open-label, single-arm phase II trial enrolling patients with MIBC (cT2–4aN0–1M0) treated with NAC followed by RC, then monitored with tumor-informed ctDNA assays.
Core strategy
- ctDNA-positive (ctDNA+) after cystectomy → start atezolizumab for up to 1 year, regardless of imaging.
- ctDNA-negative (ctDNA–) → no adjuvant immunotherapy; treat only if radiographic recurrence emerges.
Primary endpoint
- Complete response (CR) defined as ctDNA clearance + no disease on imaging at end of immunotherapy.
Follow-up
- Median follow-up ~34 months at the data lock used for this analysis.
TOMBOLA Trial
Results
ctDNA positivity was common — and often early
Among 178 evaluable patients in the intention-to-treat population:
- 58% became ctDNA+ within 2 years post-RC
- 63% of ctDNA+ cases occurred within ~4 months after RC
- Median lead-time from ctDNA detection to imaging-confirmed recurrence was ~90 days, supporting ctDNA as an earlier signal than imaging in many patients.
ctDNA reshaped who would be considered “eligible” for adjuvant immunotherapy
A clinically important finding: most ctDNA+ patients would not have met typical guideline-based selection for adjuvant immunotherapy using pathology/PD-L1 criteria alone.
- Only about half of ctDNA+ patients were “high-risk” by pathology (ypT2+ and/or N+).
- A much smaller subset were both high-risk and PD-L1–positive.
In other words, ctDNA surveillance revealed a substantial group with molecular relapse risk that conventional selection would likely miss.
Primary endpoint: substantial CR rate in ctDNA+ patients treated early
Among ctDNA+ patients who initiated atezolizumab and were evaluable for response:
- 60% achieved CR (ctDNA clearance + no evidence of disease on imaging).
Even more notable, most patients were imaging-negative at treatment start, meaning ctDNA was frequently acting as an MRD trigger rather than confirming macroscopic relapse.
TOMBOLA Trial
Outcomes strongly separated by ctDNA status
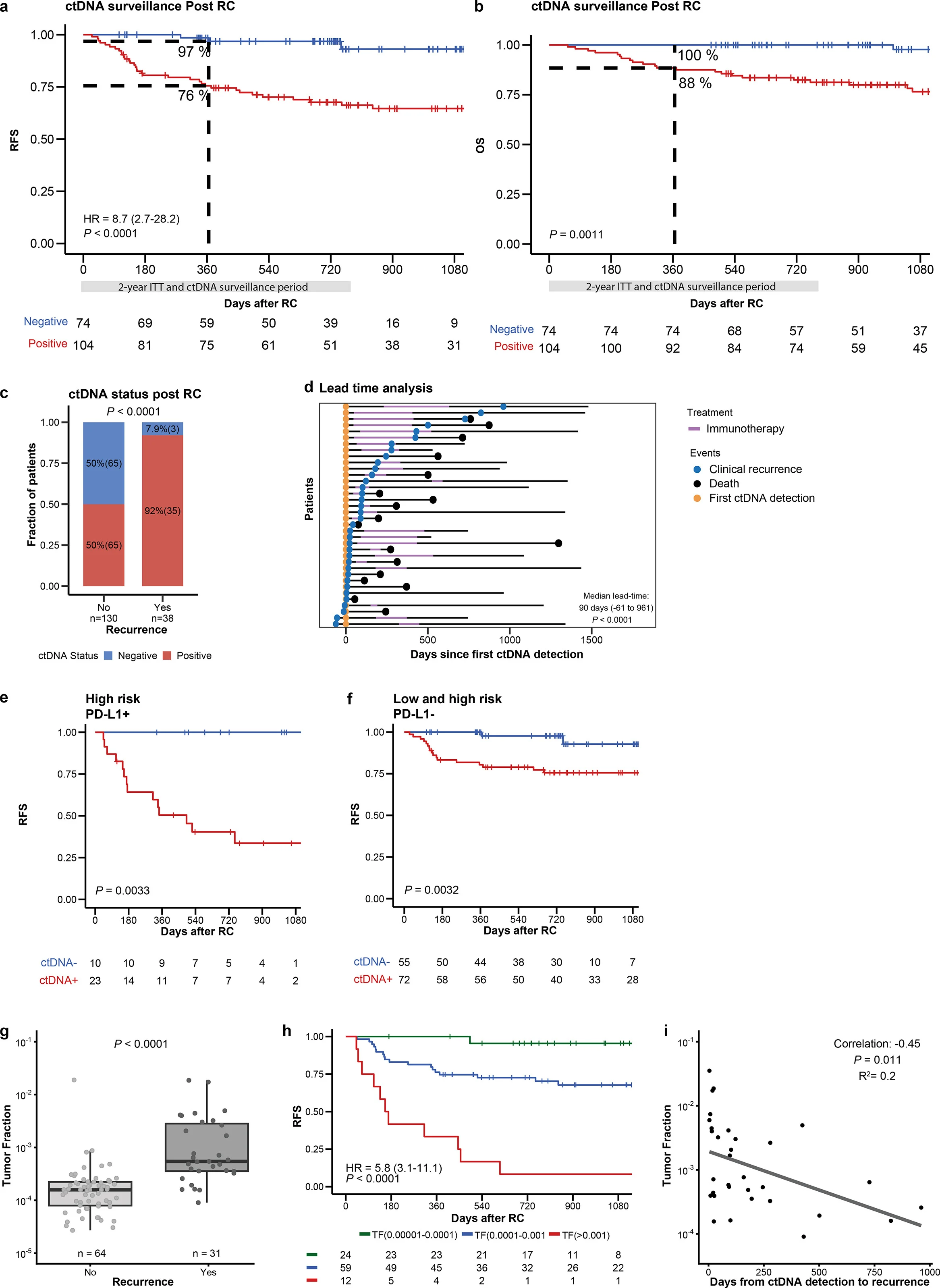
At 12 months post-RC:
- RFS ~97% in ctDNA– patients
- RFS ~76% in ctDNA+ patients (despite ctDNA-triggered immunotherapy)
This supports two parallel messages:
- ctDNA– patients did extremely well, aligning with a de-escalation approach.
- ctDNA+ patients remain higher-risk, but early immunotherapy achieved molecular and radiographic clearance in a meaningful proportion.
ctDNA dynamics looked biologically informative
Longitudinal ctDNA behavior during immunotherapy correlated with recurrence outcomes, and ctDNA “tumor fraction”at detection tracked with recurrence risk and timing—supporting the idea that ctDNA level is not just positive/negative, but may be a quantitative risk marker.
Biomarkers: What Predicted Risk and Response?
TOMBOLA reported associations between:
- ctDNA status and ctDNA level
- pathological risk group
- immune-related gene-expression signatures (including signals linked to immune biology)
A practical clinical implication is emerging: ctDNA may become the “spine” of selection, while transcriptomic immune context could refine who benefits most from early immunotherapy at MRD.
TOMBOLA Trial
Safety
Atezolizumab was described as well tolerated, with no new safety concerns reported in this setting—consistent with established experience in urothelial cancer.
Clinical Interpretation
TOMBOLA supports a precision-postoperative framework:
- Escalation: ctDNA can identify patients (including some “low-risk” by pathology) who may benefit from early immunotherapy at molecular relapse, potentially before macroscopic metastases appear.
- De-escalation: ctDNA– patients—including some with high-risk pathology—showed excellent outcomes, supporting the concept that adjuvant immunotherapy may be safely avoided in selected molecularly negative patients.
Because TOMBOLA is single-arm, it cannot fully quantify the incremental benefit of ctDNA-triggered atezolizumab versus observation; however, the risk stratification separation and the high ctDNA-negative RFS signal make the clinical logic of biomarker-guided personalization hard to ignore.
You Can Read All Article Here