Anthracyclines are long-standing components of adjuvant therapy, but in HR-positive, HER2-negative, node-negative breast cancer their added benefit is uncertain given favorable baseline outcomes and the risk of late toxicities, including cardiomyopathy and therapy-related myeloid neoplasms. With the broad adoption of the 21-gene Recurrence Score (Oncotype DX) to individualize chemotherapy, clinical practice increasingly avoids anthracyclines in lower-risk tumors.
The remaining question is whether a very high genomic risk subset benefits from adding an anthracycline to a taxane/cyclophosphamide backbone. Using patient-level data from TAILORx, this post-hoc analysis evaluates whether RS ≥ 31 identifies a chemo-sensitive cohort in which taxane-anthracycline/cyclophosphamide (T-AC) improves distant recurrence outcomes over taxane/cyclophosphamide (TC), while also exploring how benefit scales with RS, menopausal status, and tumor size. The findings aim to refine regimen selection for node-negative HR+/HER2– disease by aligning treatment intensity with genomic risk.
Title: Impact of Anthracyclines in Genomic High Risk, Node-Negative, HR-Positive/HER2-Negative Breast Cancer
Authors: N. Chen, MD, J.Q. Freeman, MPH MS, S. Yarlagadda, MD, A. Atmakuri, K. Kalinsky, MD, L. Pusztai, MD DPhil, J.A. Sparano, MD, D. Huo, MD PhD, R. Nanda, MD, F.M. Howard, MD
Published in Annals of Oncology
Background
Whether anthracyclines add meaningful benefit to adjuvant chemotherapy for early, node-negative, hormone receptor–positive (HR+)/HER2-negative breast cancer remains debated, largely because the absolute recurrence risk is low and the toxicity profile of anthracyclines (cardiotoxicity and therapy-related hematologic malignancies) is nontrivial.
Genomic assays such as the 21-gene Recurrence Score (Oncotype DX) now guide chemotherapy use, but it is unclear if very high genomic risk predicts added benefit from anthracycline-containing regimens over taxane/cyclophosphamide (TC). This post-hoc analysis of TAILORx addressed whether patients with high genomic risk (RS ≥ 31) derive superior recurrence outcomes with taxane-anthracycline/cyclophosphamide (T-AC) versus TC.
Methods
Patient-level, de-identified TAILORx data (June 2022 cut-off) were analyzed for women with stage I/II, node-negative HR+/HER2− disease and RS ≥11 who received no chemo (CMF, AC, TC, or T-AC0). The primary endpoint was distant recurrence-free interval (DRFI) in RS ≥31 comparing T-AC vs TC. Secondary endpoints: DRFS, RFI, RFS, DFS, OS. Multivariable Cox models adjusted for age, tumor size, grade, ER/PR status, and RS; interaction tests assessed differential benefit by RS ≥31. Restricted cubic splines estimated treatment effect as a continuous function of RS. RSClin was explored as an alternative predictor of anthracycline benefit.
Study population
Of 7,789 eligible cases: 2,111 received TC, 438 T-AC, 1,152 AC, 247 CMF, and 3,841 no chemotherapy. Those given T-AC were younger, had larger/higher-grade tumors, more PR-negative disease, and higher RS (mean RS 30 vs 23 for TC).
Results
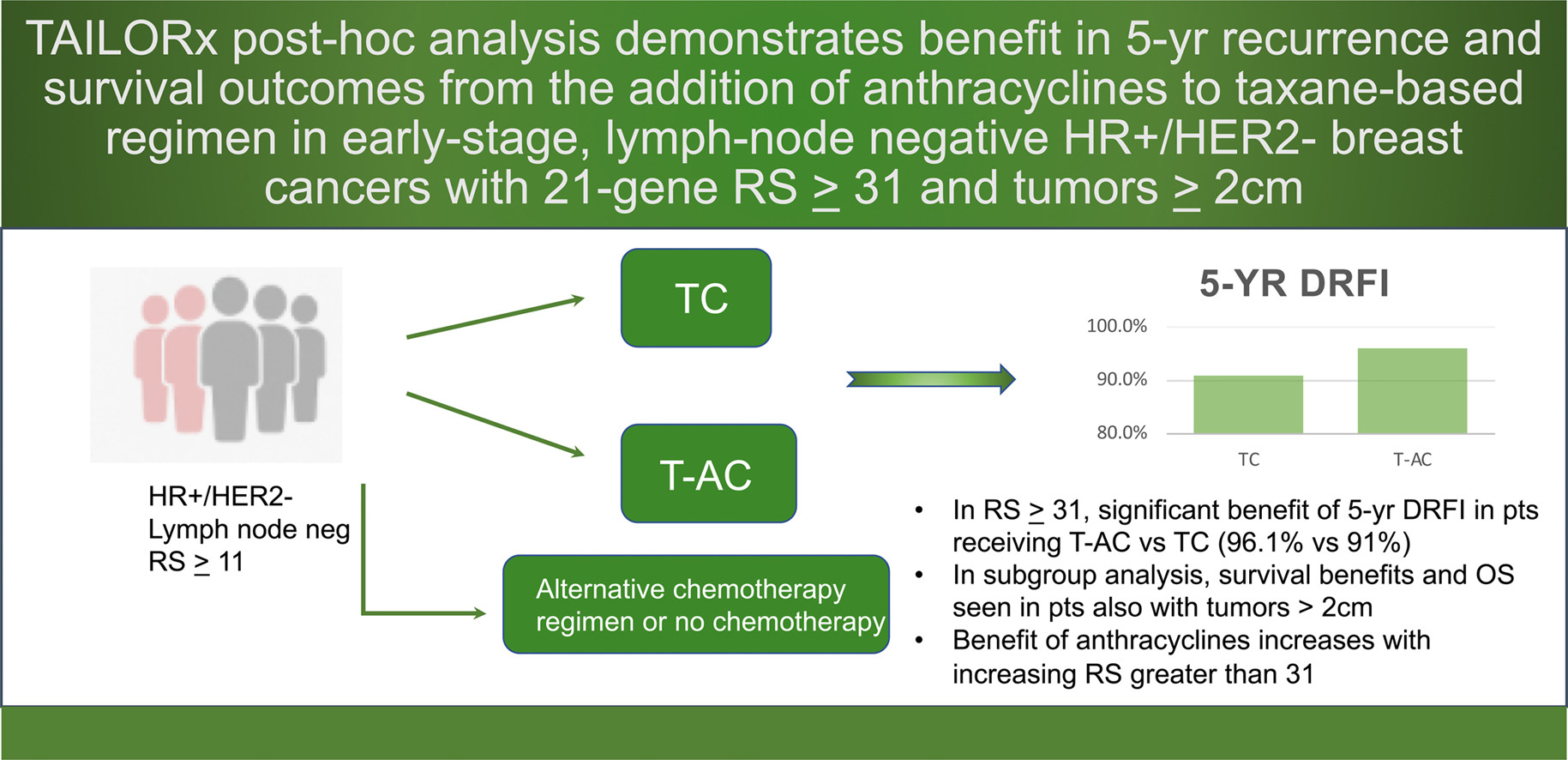
Adjusted efficacy outcomes comparing T-AC versus TC are reported by 21-gene Recurrence Score, with the primary analysis evaluating DRFI in the high-risk subgroup (RS ≥ 31).
Primary analysis (RS ≥31):
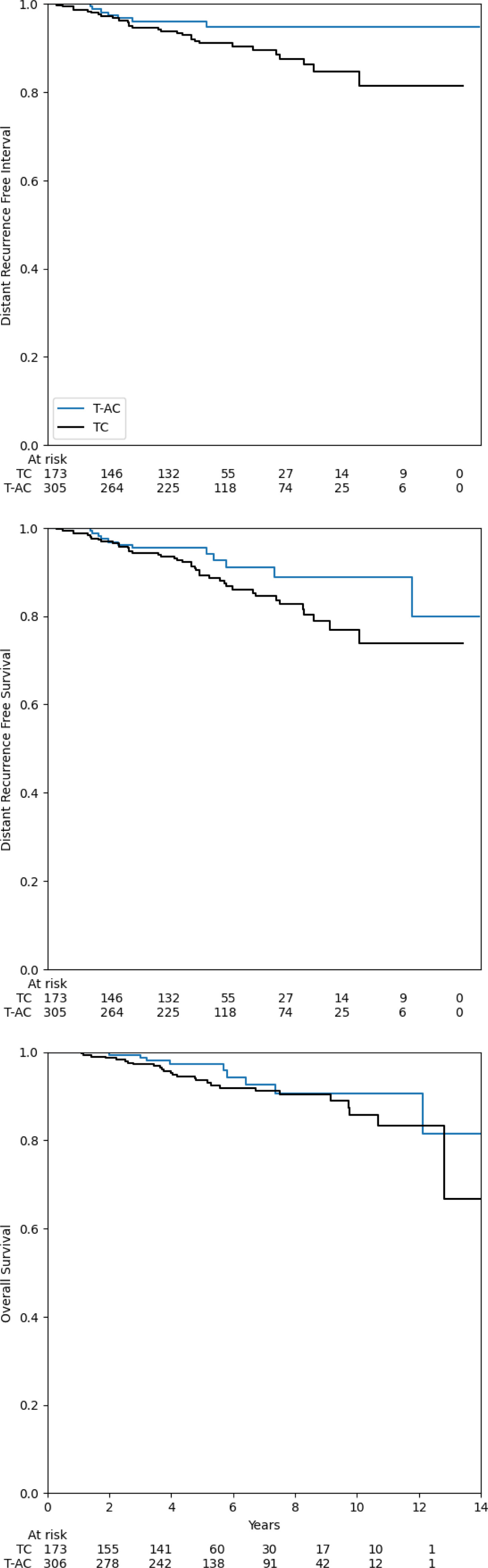
- DRFI (5-yr): 96.1% with T-AC vs 91.0% with TC; aHR 0.31, P=0.006
- DRFS (5-yr): 95.5% vs 89.3%; aHR 0.49, P=0.032
- OS (5-yr, adjusted): 97.3% vs 93.6%; aHR 0.67, P=0.31 (trend, not significant).
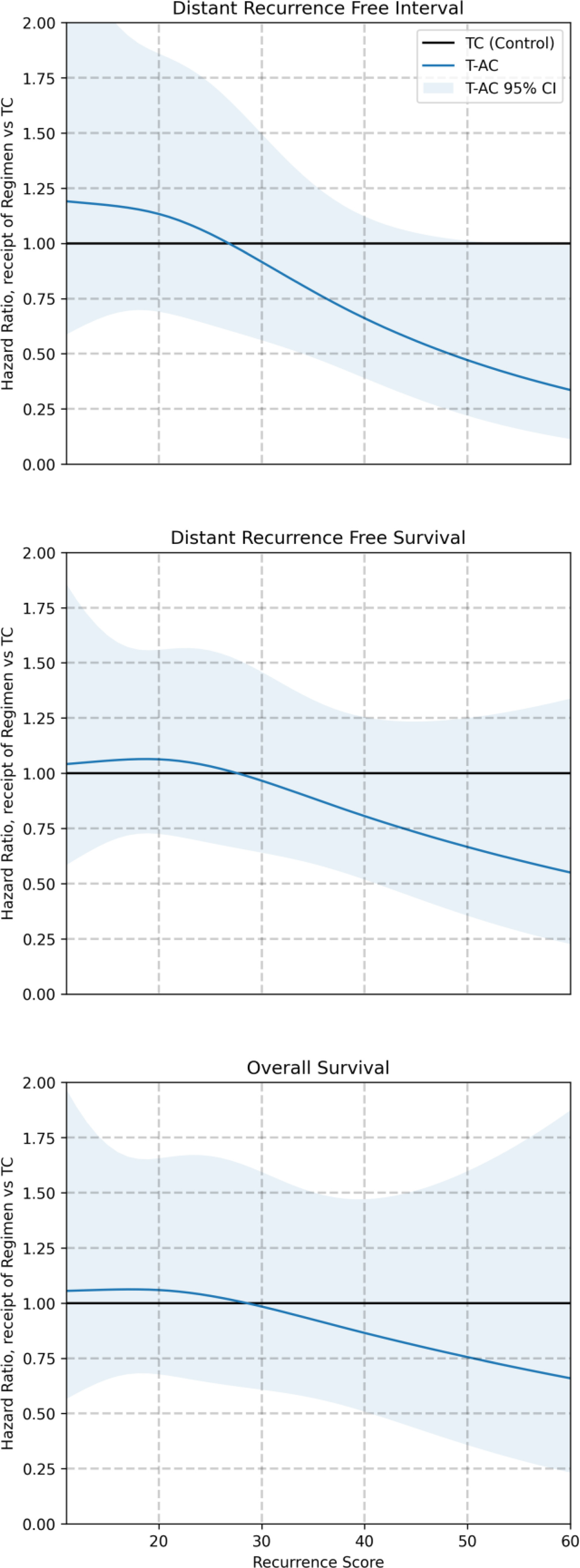
- Significant treatment-by-RS interaction supported preferential benefit of T-AC when RS ≥31; no benefit for RS <31 across endpoints.
Other regimens in RS ≥31:
- AC alone showed no advantage over TC.
- CMF was associated with worse DRFI (aHR 2.99; P=0.047) in this high-RS subset.
- No chemotherapy and CMF performed worse as RS increased.
Menopausal status (RS ≥31):
- DRFI benefit with T-AC vs TC was seen in both premenopausal (aHR 0.19; P=0.022) and postmenopausal (aHR 0.25; P=0.025) patients.
Tumor size:
- Benefit signals were consistent across subgroups, except T1 tumors, where no clear advantage for T-AC was observed. The discussion highlights a stronger effect in tumors >2 cm.
RS 26–30:
- No interaction indicating benefit for any specific regimen versus TC.
RSClin:
A ≥10% predicted chemo-benefit threshold trended toward better DRFI with T-AC vs TC but did not reach significance, suggesting RS alone (at high levels) may better capture anthracycline sensitivity.
Key findings
- In node-negative HR+/HER2− disease, anthracycline + taxane (T-AC) improves 5-year DRFI and DRFS over TC only when RS ≥31; no benefit for RS <31.
- OS shows a favorable trend but is not statistically significant at 5 years.
- AC alone does not outperform TC; CMF underperforms in RS ≥31.
- Benefit is seen irrespective of menopausal status and appears more relevant for tumors >2 cm.
Clinical implications
For the small minority of node-negative HR+/HER2− patients with RS ≥31—especially with T2 tumors—adding an anthracycline to a taxane regimen can reduce distant recurrence at 5 years versus TC. For RS 26–30 or small (T1) tumors, the incremental benefit is uncertain and should be weighed against anthracycline risks (cardiotoxicity, therapy-related myeloid neoplasms). AC alone does not substitute for T-AC in this context.
Limitations
Non-randomized regimen selection within TAILORx introduces confounding, mitigated but not eliminated by adjustment. RS ≥31 cases are uncommon; subgroup estimates are therefore imprecise. Compliance data for endocrine therapy were unavailable. Node-positive disease was not included, limiting generalizability.
Conclusion
In TAILORx patients with node-negative, HR+/HER2- breast cancer, very high genomic risk (RS ≥ 31) identifies a chemo-sensitive subgroup that experiences fewer distant recurrences when an anthracycline is added to a taxane/cyclophosphamide backbone. The benefit was not observed for RS < 31, supporting an anthracycline-sparing approach (TC) for the majority of genomically lower-risk tumors. Signals were strongest in larger tumors, consistent with higher baseline risk and potential for greater absolute benefit. Importantly, regimen choice in TAILORx was not randomized, and T-AC recipients had less favorable baseline features, necessitating adjusted analyses; nonetheless, the interaction between high RS and T-AC benefit remained significant.
Given the known late toxicities of anthracyclines, clinicians should incorporate RS level, tumor size, patient comorbidities, and preferences into shared decision-making. Prospective validation that randomizes anthracycline use within high-RS populations—and integrates modern endocrine intensification (e.g., adjuvant CDK4/6 inhibition for selected T2N0)—is warranted to refine patient selection and quantify long-term net benefit.
You can read the full article here.


