The management of Bacillus Calmette-Guérin (BCG)–unresponsive high-risk non–muscle-invasive bladder cancer (NMIBC) remains a significant clinical challenge. Radical cystectomy remains the gold standard, but many patients pursue bladder-preserving systemic or intravesical therapies despite a high risk of recurrence and disease progression.
However, the lack of validated biomarkers to guide treatment selection and monitor therapeutic response limits precision in managing these patients.
Urine tumor DNA (utDNA) analysis offers a non-invasive approach to detect minimal residual disease (MRD) and assess recurrence risk. The UroAmp assay—a multigene next-generation sequencing (NGS) platform detecting somatic mutations, copy-number variations, and aneuploidy—had previously shown predictive utility in BCG-naïve NMIBC.
This study presents a correlative biomarker analysis of the SWOG S1605 phase II trial, which evaluated atezolizumab (anti-PD-L1) in BCG-unresponsive NMIBC, testing whether utDNA profiling at baseline and 3 months could predict treatment response and long-term outcomes.
Study Design and Methods
The analysis was conducted within the SWOG S1605 trial (NCT02844816) — a single-arm, multicenter phase II study evaluating atezolizumab in patients with high-risk, BCG-unresponsive non–muscle-invasive bladder cancer (NMIBC).
A total of 129 eligible patients were enrolled, all of whom had carcinoma in situ (CIS) with or without Ta/T1 tumorsafter complete transurethral resection of visible disease.
Patients received intravenous atezolizumab administered every three weeks as systemic immunotherapy.
The study had two primary efficacy endpoints:
- Complete response (CR) at 6 months for patients with CIS at baseline.
- Event-free survival (EFS) at 18 months for the overall study population.
For biomarker assessment, urine tumor DNA (utDNA) was analyzed using the UroAmp assay at two time points — baseline (n = 89) and 3 months (n = 77). The test quantified genomic alterations and aneuploidy patterns from urinary DNA.
All utDNA results were blinded to investigators and subsequently correlated with clinical outcomes using Cox proportional hazards regression and Kaplan–Meier survival analyses, with adjustments made for baseline CIS status.
Results
Baseline utDNA as a Predictor of Treatment Response and Recurrence
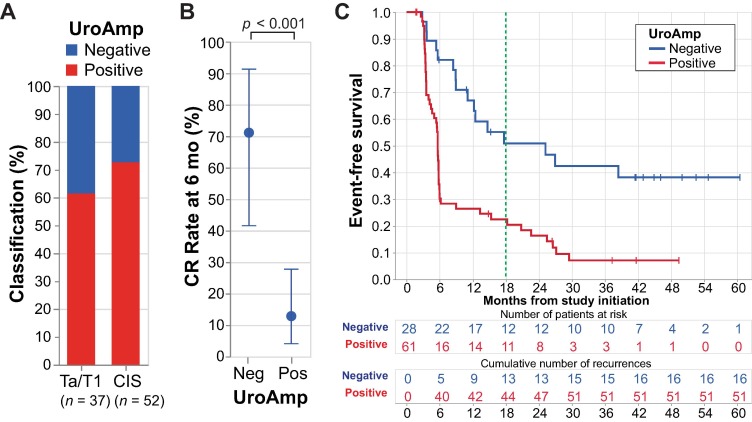
At the start of treatment, 69% (61 of 89) patients tested UroAmp-positive for urine tumor DNA (utDNA).
This baseline status strongly correlated with treatment outcomes:
Complete Response (CR) at 6 months
- UroAmp-positive: 13%
- UroAmp-negative: 71%
- p < 0.001
18-month Event-Free Survival (EFS)
- UroAmp-positive: 23%
- UroAmp-negative: 51%
- Hazard Ratio (HR): 2.8; p < 0.001
These findings indicate that patients with detectable utDNA before treatment were significantly less likely to achieve a complete response or remain disease-free.
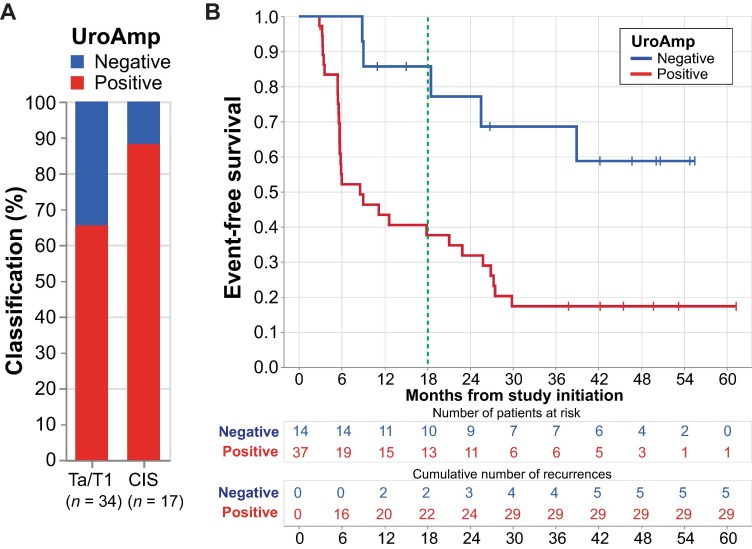
Three-Month utDNA Further Stratifies Recurrence Risk
Among 51 patients who showed no clinical evidence of disease at 3 months, utDNA status remained a powerful prognostic marker:
18-month EFS:
- UroAmp-positive: 38%
- UroAmp-negative: 86%
- HR: 3.5; p = 0.012
CR in the CIS subgroup:
- UroAmp-positive: 47%
- UroAmp-negative: 100%
Thus, persistence of utDNA after 3 months of atezolizumab treatment identified patients at higher risk of recurrence despite no visible disease.
Comparison with Cytology
At the 3-month evaluation point:
- Cytology demonstrated slightly higher overall accuracy (72%),
but a lower negative predictive value than UroAmp. - UroAmp achieved 100% sensitivity and 100% negative predictive value,
making it particularly valuable for detecting microscopic or occult disease not visible on cystoscopy.
Interpretation
This study demonstrates that utDNA profiling at baseline and early on-treatment (3 months) can effectively stratify patients according to risk of recurrence following atezolizumab therapy.
Baseline utDNA positivity identified patients with a low likelihood of complete response and early disease recurrence.
Persistent utDNA positivity at 3 months, even in clinically disease-free patients, predicted inferior 18-month outcomes, suggesting molecular detection of residual disease precedes radiographic or cystoscopic relapse.
These results parallel the prognostic performance of plasma circulating tumor DNA (ctDNA) in advanced disease and reinforce utDNA as a biologically relevant biomarker of immunotherapy response.
Limitations
- Retrospective biomarker analysis within a single-arm trial (no comparator arm).
- Small cohort size (n = 98 for utDNA testing).
- Variability in sample handling across sites (no buffer addition, potential for white blood cell DNA contamination).
- Misclassification in a small subset of samples, corrected in sensitivity analyses.
- Limited 60-gene panel may not capture all genomic alterations in NMIBC.
Clinical Implications
The integration of urine tumor DNA profiling could transform how bladder-preserving therapies are selected and monitored in BCG-unresponsive NMIBC.
High utDNA burden identifies patients unlikely to benefit from PD-(L)1 therapy and may warrant early cystectomy or alternative immunotherapy combinations.
utDNA negativity during therapy indicates deep molecular remission and supports continued bladder preservation.
Future trials should evaluate utDNA as a real-time decision-making tool, guiding escalation or de-escalation of treatment and serving as an intermediate endpoint for early efficacy assessment.
Key Takeaway Messages
- UroAmp utDNA is a reliable, non-invasive biomarker for risk stratification and molecular response monitoring in BCG-unresponsive NMIBC treated with immunotherapy.
- Baseline positivity correlates with poor 6-month CR and 18-month EFS.
- Persistent utDNA positivity at 3 months signals a high recurrence risk even in clinically disease-free patients.
- Incorporating utDNA into clinical trial design could refine patient selection and optimize sequencing of bladder-preserving strategies.
You Can Read All Article Here


