SunRISe-1 Study evaluates novel treatment strategies for high-risk non–muscle-invasive bladder cancer (NMIBC) that recurs despite adequate bacillus Calmette-Guérin (BCG) therapy—one of the most challenging clinical scenarios in uro-oncology. While radical cystectomy remains the standard of care, it carries substantial morbidity, mortality, and long-term quality-of-life consequences. Current bladder-sparing alternatives, including pembrolizumab, nadofaragene firadenovec, and NAI+BCG, yield only modest complete response rates and frequently involve systemic or immune-related toxicities.
TAR-200, a first-in-class intravesical gemcitabine-releasing drug-delivery system, was developed to provide sustained local chemotherapy exposure with minimal systemic effects. The phase IIb SunRISe-1 trial investigated three strategies—TAR-200 alone, TAR-200 combined with the PD-1 inhibitor cetrelimab, and cetrelimab monotherapy—to determine whether continuous intravesical gemcitabine could outperform systemic immunotherapy in BCG-unresponsive high-risk NMIBC.
You Can Also Read About Bladder Cancer: Symptoms ,Causes, Stages, Diagnosis and Treatment
Study Design
SunRISe-1 (NCT04640623) was a parallel-cohort phase IIb study enrolling:
- Cohort 1 (C1): TAR-200 + cetrelimab
- Cohort 2 (C2): TAR-200 monotherapy
- Cohort 3 (C3): Cetrelimab monotherapy
- Cohort 4 (C4): TAR-200 monotherapy for high-risk papillary-only NMIBC
Treatment duration:
- TAR-200 every 3 weeks → then every 12 weeks (up to 24 months)
- Cetrelimab every 3 weeks (up to 18 months)
Primary endpoints:
- Cohorts 1–3: Centrally confirmed complete response (CR)
- Cohort 4: Disease-free survival (DFS)
Key Results
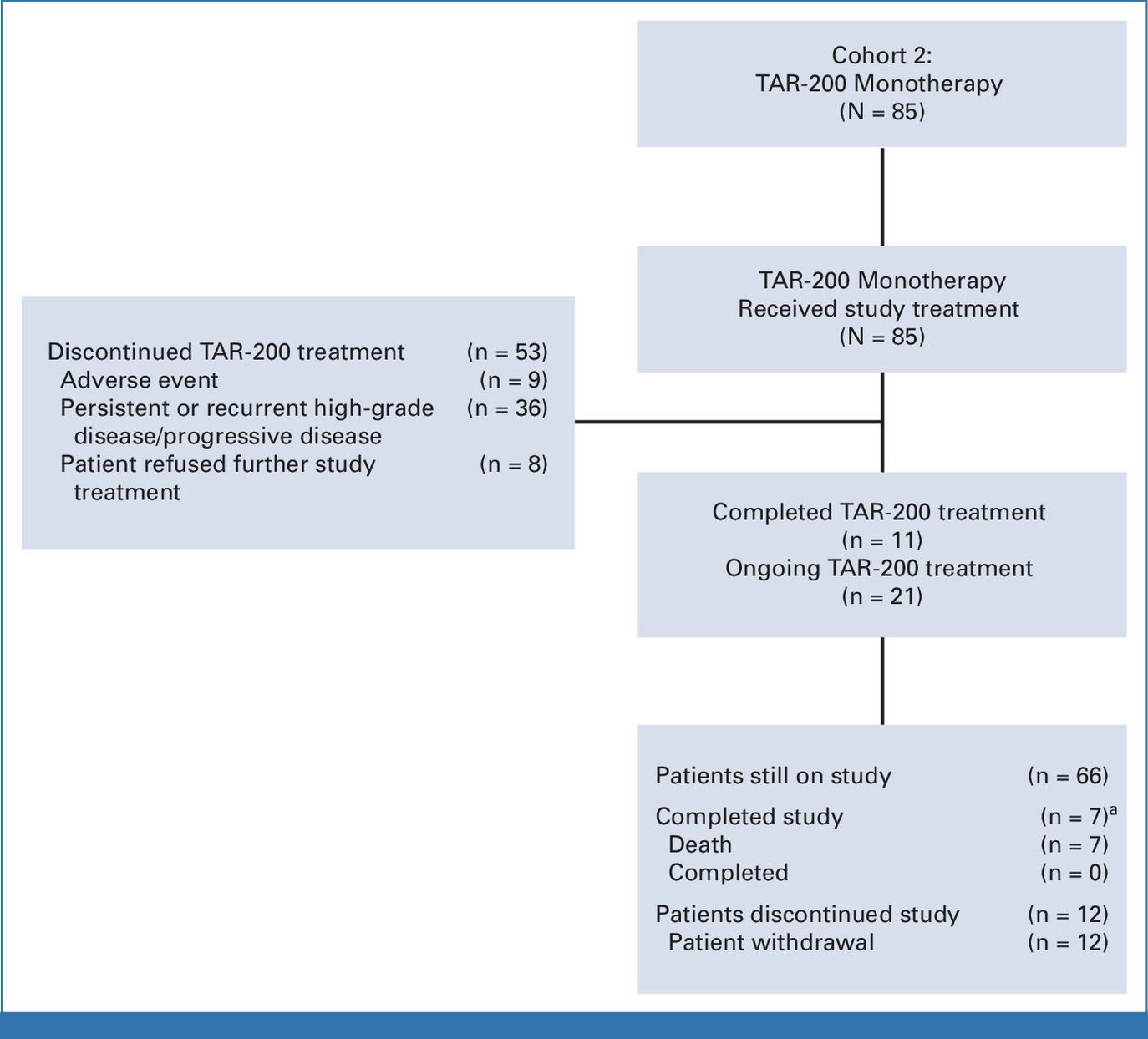
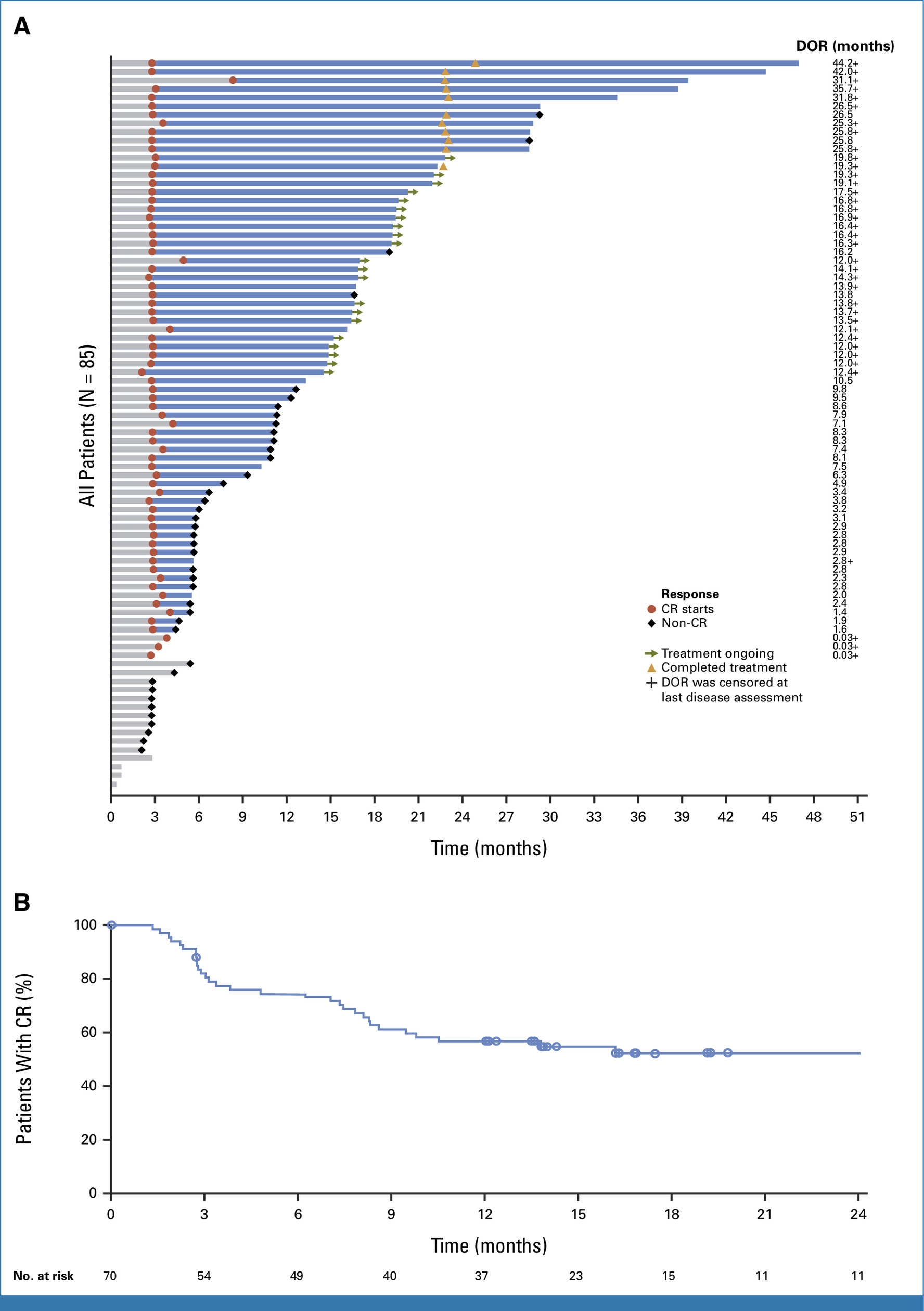
TAR-200 Monotherapy: Highest CR Rate Ever Reported
Cohort 2: CIS ± papillary disease
- Complete response (CR): 82.4% (95% CI, 72.6–89.8)
- Median time to CR: 2.8 months
- Median DOR: 25.8 months
- 12-month CR rate: 45.9%
- Ongoing responses: 33 of 70 responders (47.1%)
- 12-month cystectomy-free rate: 86.6%
Interpretation: This represents the highest single-agent CR rate ever achieved in BCG-unresponsive CIS, with durable responses and strong bladder preservation.
TAR-200 in Papillary-Only High-Risk NMIBC (Cohort 4)
- 6-month DFS: 85.3%
- 9-month DFS: 81.1%
- 12-month DFS: 70.2%
- Median DFS: Not reached
Interpretation: These represent the best DFS outcomes reported to date for papillary-only BCG-unresponsive NMIBC.
TAR-200 + Cetrelimab Combination (Cohort 1)
- CR rate: 67.9%
- Median DOR: Not reached
- 12-month DOR rate: 76.3%
- Toxicity: Higher than TAR-200 alone
Interpretation: Although active, the combination produces more toxicity without improving outcomes compared to TAR-200 monotherapy.
Cetrelimab Monotherapy (Cohort 3)
- CR rate: 46.4%
- Median DOR: 8.6 months
Interpretation: Efficacy consistent with other PD-1 inhibitors in NMIBC, but lower than both TAR-200 monotherapy and combination therapy.
Safety
Across the SunRISe-1 study, TAR-200 demonstrated a generally favorable safety profile, particularly when used as monotherapy. Most adverse events associated with TAR-200 alone were mild to moderate and localized to the lower urinary tract. In the TAR-200 monotherapy cohort, treatment-related adverse events occurred in 83.5% of patients, with the majority presenting as urinary symptoms such as pollakiuria (43.5%), dysuria (40%), urinary urgency (24.7%), and urinary tract infections (21.2%). Severe toxicity was uncommon: grade ≥3 events were reported in only 12.9% of patients, and serious adverse events occurred in 5.9%. Importantly, no treatment-related deaths were observed, and most urinary symptoms were short-lived and managed conservatively. Overall, TAR-200 monotherapy was well tolerated, with a safety profile appropriate for a bladder-sparing therapy.
In contrast, the combination of TAR-200 with the PD-1 inhibitor cetrelimab resulted in substantially higher toxicity. Grade ≥3 adverse events occurred in 37.7% of patients receiving combination therapy, and immune-related toxicities were common, reflecting the systemic effects of checkpoint inhibition. Despite the intensified toxicity, combination therapy did not meaningfully improve complete response rates compared with TAR-200 alone, resulting in a less favorable risk–benefit profile.
Cetrelimab monotherapy also demonstrated acceptable tolerability but produced a different toxicity pattern typical of PD-1 inhibitors. Grade ≥3 adverse events occurred in 7.1% of patients, and one case of immune-mediated myopericarditis was reported. Although less toxic than the combination arm, cetrelimab alone produced lower efficacy outcomes compared with TAR-200 monotherapy.
Interpretation
TAR-200 monotherapy demonstrated:
- The highest CR rate ever reported in BCG-unresponsive CIS
- Durable responses (median DOR 25.8 months)
- Excellent DFS in papillary-only disease
- Strong bladder-preserving outcomes
- A favorable safety profile
- Minimal systemic toxicity
These findings support TAR-200 as a promising bladder-sparing treatment option, addressing a major unmet need for patients who are unwilling or ineligible for radical cystectomy.
Clinical Significance
- TAR-200 may shift clinical practice as a novel intravesical sustained-release therapy.
- Results emphasize that local continuous gemcitabine exposure may outperform systemic checkpoint inhibitors in this setting.
- Ongoing trials (SunRISe-2, -3, and -4) will define its role across NMIBC risk groups and earlier-stage disease.
You Can Read All Article Here