Over the past decade, immunotherapy has dramatically reshaped the treatment landscape for melanoma, one of the most aggressive forms of skin cancer. Once associated with poor prognosis in advanced stages, melanoma now represents one of immunotherapy’s greatest success stories. But how effective is it, and what do patients need to know?
This article explores the success rate of immunotherapy for melanoma, with a focus on FDA-approved therapies like immune checkpoint inhibitors, combination regimens, and long-term survival data from major clinical trials—all based on human studies and evidence-based medical journals.
Read About Melanoma on OncoDaily
Understanding Melanoma and the Role of Immunotherapy
Melanoma arises from melanocytes, the pigment-producing cells of the skin. While early-stage melanoma can often be cured with surgery, advanced melanoma was historically difficult to treat, with five-year survival rates under 20% before immunotherapy was introduced.
Immunotherapy has changed this trajectory by activating a patient’s immune system to recognize and kill melanoma cells. The two main types of immunotherapy for melanoma are:
- Immune checkpoint inhibitors: Anti-PD-1 (nivolumab, pembrolizumab) and anti-CTLA-4 (ipilimumab) antibodies
- Adoptive cell therapy: Such as tumor-infiltrating lymphocytes (TILs) or experimental CAR T-cell therapies (still investigational)
Single-Agent Immunotherapy: What Does the Data Say?
Checkpoint inhibitors have been approved as first-line treatments for unresectable or metastatic melanoma. Multiple phase III trials support their efficacy:
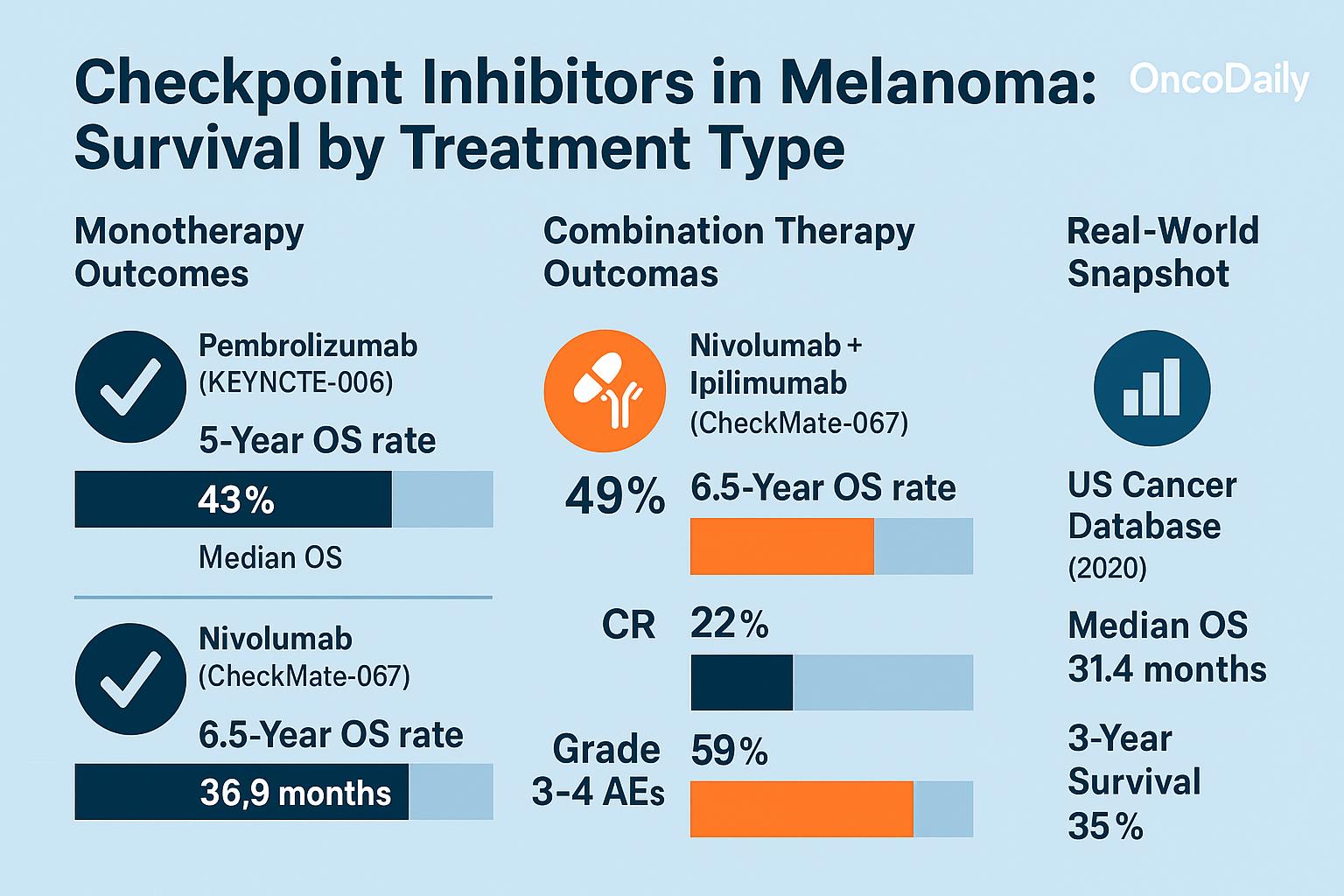
- The KEYNOTE-006 trial demonstrated that pembrolizumab improved 2-year overall survival (OS) to 55% compared to 43% with ipilimumab (Robert et al., NEJM, 2015).
- In the CheckMate 067 trial, nivolumab alone resulted in a 5-year OS of 44% in advanced melanoma patients (Larkin et al., NEJM, 2019).
These outcomes reflect a major improvement over the pre-immunotherapy era, where median survival was often less than 1 year.
Combination Immunotherapy: Nivolumab + Ipilimumab
Combining PD-1 and CTLA-4 inhibitors has further improved outcomes, albeit with increased toxicity.
- In CheckMate 067, the combination of nivolumab and ipilimumab achieved a 5-year OS of 52% in metastatic melanoma patients, compared to 44% with nivolumab alone and 26% with ipilimumab alone (Larkin et al., 2019).
- Progression-free survival (PFS) was also significantly higher with the combination: 11.5 months vs. 6.9 months with nivolumab alone.
While the combination therapy offers higher success rates, grade 3–4 immune-related adverse events (irAEs) occurred in over 55% of patients, highlighting the need for careful patient selection and monitoring.
Neoadjuvant and Adjuvant Settings
Immunotherapy for melanoma is increasingly being used in earlier stages of the disease, either before surgery (neoadjuvant) or after surgery (adjuvant) to reduce the risk of recurrence
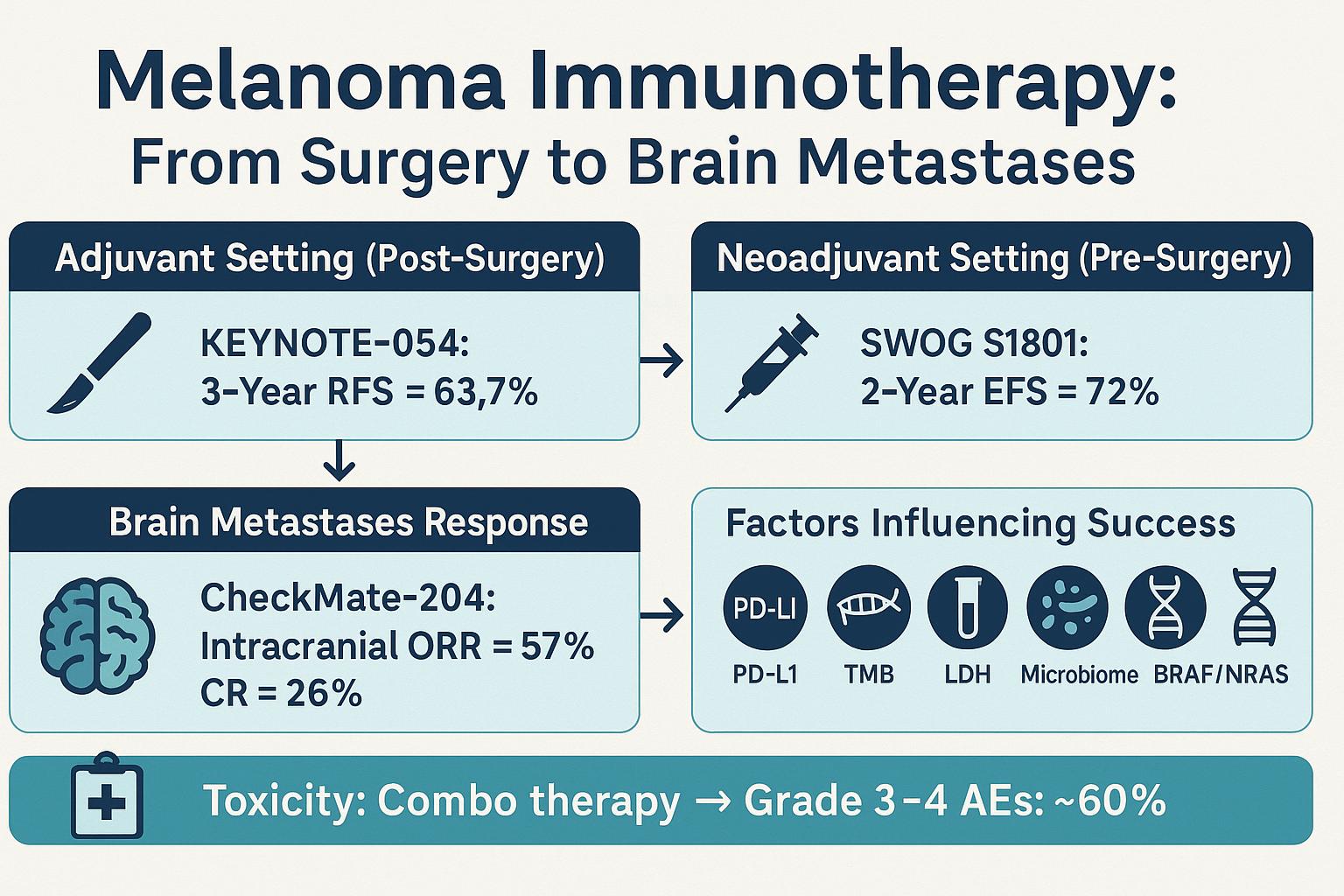
- KEYNOTE-716 showed that adjuvant pembrolizumab significantly improved recurrence-free survival in Stage IIB/C melanoma, with 12-month recurrence-free rates of 90.5% vs. 83.1% with placebo (Eggermont et al., NEJM, 2022).
- Neoadjuvant trials such as PRADO and OpACIN have shown promising immune responses, with major pathologic response (MPR) rates exceeding 60%, suggesting a durable immune priming effect before surgery.
Long-Term Survivors and the Possibility of Cure
One of the most exciting aspects of immunotherapy for melanoma is the emergence of long-term survivors. Follow-up data from CheckMate 067 show that more than half of patients on combination therapy are alive 5 years later, and a subset appears to be functionally cured.
Studies now routinely report plateauing survival curves, where a proportion of patients remain disease-free off therapy—a phenomenon rarely seen in metastatic solid tumors before immunotherapy.
For example:
- A 2024 pooled analysis by Ascierto et al. showed that patients achieving complete response (CR) after immunotherapy have a 5-year OS of over 85%, with many remaining off treatment.
Who Benefits Most—and Who Doesn’t?
Not all patients respond to immunotherapy. Predictors of success include:
- Tumor PD-L1 expression (though not a definitive biomarker)
- Tumor mutational burden (TMB)
- Pre-existing T-cell infiltration in the tumor
- Genomic subtype (e.g., BRAF mutation status)
Conversely, patients with liver metastases, high lactate dehydrogenase (LDH) levels, or immune-suppressive tumor microenvironments tend to have lower response rates. Efforts are underway to improve outcomes in these patients via triplet therapy, personalized vaccines, and novel immune agonists.
Real-World Data: Confirming the Success Rates
Population-based registries and real-world cohorts mirror clinical trial results. For example:
- An analysis from the SEER-Medicare database (2023) reported that the median OS in real-world metastatic melanoma patients treated with immunotherapy is now over 25 months—a stark contrast to pre-2011 data.
- Real-world complete response rates remain around 15–20%, with stable disease or partial responses in over 40% of patients.
These data support the generalizability of trial results, although access disparities, insurance limitations, and performance status exclusions still limit global reach.
What About Stage IV Melanoma with Brain Metastases?
Melanoma is one of the few solid tumors where immunotherapy shows meaningful activity in the brain:
- The CheckMate 204 trial showed response rates over 50% in asymptomatic brain metastases with nivolumab + ipilimumab.
- Median OS exceeded 24 months, a dramatic shift from the historical 4–6 month survival for patients with CNS disease.
Success Rate Summary Across Settings
The success of immunotherapy for melanoma varies depending on the stage of the disease, the type of immunotherapy used, and patient-specific factors such as genetic mutations or immune profile. In early-stage (resectable) melanoma, immunotherapy is increasingly used as an adjuvant treatment after surgical removal of the tumor to reduce the risk of recurrence. In this setting, clinical trials have shown that immune checkpoint inhibitors such as nivolumab can significantly improve relapse-free survival. For example, in the CheckMate 238 trial, patients with stage III and IV resected melanoma who received nivolumab had a 3-year recurrence-free survival rate of approximately 58%, compared to 39% with ipilimumab (Weber et al., 2020).
In the metastatic setting—where melanoma has spread to other parts of the body—the success of immunotherapy has been especially notable. Combination immunotherapy using nivolumab and ipilimumab has led to durable responses in a subset of patients. The 5-year overall survival rate in the CheckMate 067 trial was reported at 52% for the combination, 44% for nivolumab alone, and 26% for ipilimumab alone (Larkin et al., 2019). These numbers represent a remarkable improvement over historical survival rates for advanced melanoma, which were typically less than 10% before the immunotherapy era.
Patients with specific biomarkers, such as high tumor mutational burden (TMB) or PD-L1 expression, may experience even higher response rates. However, it is important to note that not all patients respond to immunotherapy, and some may experience immune-related adverse events that require early treatment discontinuation or management with immunosuppressants.
In summary, while immunotherapy does not guarantee success for every melanoma patient, it has transformed outcomes in both early and advanced stages of the disease. Long-term survival is now achievable for a significant proportion of patients, particularly those receiving combination checkpoint inhibitors in the metastatic setting or adjuvant therapy after surgery in high-risk early-stage disease.
Limitations and Ongoing Research
Despite remarkable success, immunotherapy still fails to benefit 30–50% of patients. Key challenges include:
- Primary resistance mechanisms
- Immune escape and tumor plasticity
- Toxicity management (e.g., colitis, pneumonitis, endocrinopathies)
Ongoing clinical trials are exploring next-generation checkpoint inhibitors, bispecific antibodies, and personalized mRNA vaccines for melanoma to further increase the response rate and durability.
Final Thoughts: A Patient’s Guide
For patients considering immunotherapy, here’s what to keep in mind:
Immunotherapy is not a one-size-fits-all treatment; response rates vary depending on stage, biology, and prior therapies. Discussions with your oncologist should include PD-L1 status, BRAF mutation testing, and potential side effects. Clinical trials remain an excellent option, especially for non-responders or patients with rare subtypes.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


