At SABCS 2025, Dr. Erica Mayer delivered a sweeping and forward-looking overview of endocrine-based therapy for HR+/HER2– metastatic breast cancer (MBC). Her central message resonated across the packed session hall: the era of endocrine monotherapy is ending, and the field is transitioning toward precision-guided, targeted combinations informed by genomic profiling and early detection of resistance.
Oral SERDs Set the Foundation for the Next Treatment Era
Dr. Mayer began with the evolution of oral selective estrogen receptor degraders (SERDs)—a class designed to overcome the pharmacologic limitations of fulvestrant. Four leading agents—elacestrant, imlunestrant, giredestrant, and camizestrant—have been evaluated across a broad pipeline of Phase II and III trials.
EMERALD: Proof of Concept for Oral SERD Monotherapy
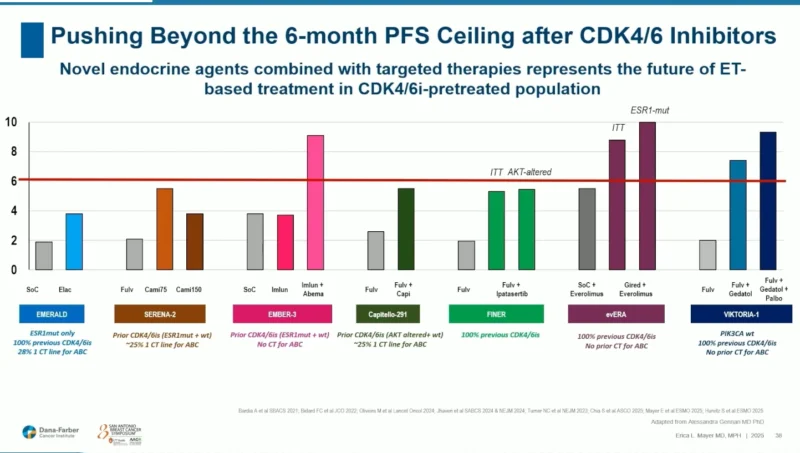
The EMERALD Phase III trial established the clinical relevance of oral SERDs. Patients previously treated with endocrine therapy and CDK4/6 inhibitors were randomized to elacestrant monotherapy or investigator’s-choice endocrine therapy.
The benefit was most pronounced in tumors with ESR1 mutations, where elacestrant improved progression-free survival (PFS) and supported the first FDA approval of an oral SERD (2023). However, Dr. Mayer highlighted a notable curve pattern: a steep early drop in PFS, signaling a subset of deeply endocrine-resistant patients unlikely to benefit from monotherapy.
This observation set the stage for why combinations may offer superior outcomes.
EMBER-3: SERD + CDK4/6 Inhibitor
The EMBER-3 study evaluated imlunestrant both as monotherapy and in combination with abemaciclib.
While monotherapy again showed its strongest activity in ESR1-mutant disease, the true breakthrough appeared in the combination arm, where PFS extended beyond what monotherapy alone had ever achieved in this setting.
Critically, the combination benefit was seen regardless of ESR1 mutation status, suggesting that pairing SERDs with agents addressing parallel resistance pathways may be essential to overcoming endocrine escape.
Updated results of EMBER-3 will be presented later this week at SABCS, generating significant anticipation.
EVERA: SERD + mTOR Inhibition
The EVERA Phase III trial compared giredestrant + everolimus versus standard endocrine therapy + everolimus.Unlike earlier SERD studies, the control arm here used active combination therapy, making this a more modern comparator.
EVERA met both primary endpoints, showing prolonged PFS in the intent-to-treat population and in ESR1-mutant tumors. Exploratory data suggested minimal benefit in the ESR1-wild-type subgroup, underscoring the nuanced role of molecular selection.
A biomarker-driven analysis of EVERA will be presented during General Session 3 at SABCS.
Up to 15% of patients harbor concurrent ESR1 and PIK3CA mutations.Dr. Mayer highlighted subset analyses from EMERALD and EMBER-3 showing that SERD-based regimens—especially in combination—retain activity across these co-mutated tumors. This reinforces the role of SERDs as a backbone therapy in genomically complex disease.
SERENA-6 Trial
SERENA-6 is an innovative study, which tested whether early molecular intervention—triggered not by clinical progression but by the appearance of an ESR1 mutation on serial ctDNA—could delay progression.
In this two-step trial design, more than 3,000 patients on first-line AI + CDK4/6 therapy underwent frequent ctDNA monitoring. When an ESR1 mutation emerged without radiographic progression, patients were randomized to switch from the AI to camizestrant or continue standard therapy.
The early switch prolonged PFS and notably delayed deterioration in quality of life, including pain, fatigue, dyspnea, and emotional well-being.
This study is the first large-scale demonstration that ctDNA-guided endocrine switching may intercept resistance before it manifests clinically.
Updated ctDNA analyses from SERENA-6 will be released in the Rapid Fire session.
Beyond SERDs: New ER-Targeted Classes
Dr. Mayer also previewed additional ER-targeted strategies progressing through the pipeline:
- PROTAC ER degraders such as vepdegestrant, which showed superior PFS versus fulvestrant in ESR1-mutant disease in the VIKTORIA-2 trial.
- Complete estrogen receptor antagonists (CERANs) like palazestrant, with early signals of activity both alone and in combination with CDK4/6 inhibitors.
- Next-generation SERMs such as lasofoxifene, currently in Phase III evaluation.
- These agents expand the landscape beyond traditional endocrine therapy and offer multiple, non–cross-resistant mechanisms of action.
Safety: Predictable and Manageable
Across trials, oral SERDs have shown consistent tolerability, with mainly low-grade gastrointestinal symptoms.Some agents produce characteristic but non-impactful effects—such as photopsia with camizestrant or low-grade bradycardia in certain compounds—but these rarely affect daily life or lead to discontinuation.Combination regimens inherit toxicity from their partner drugs, but SERDs themselves remain relatively clean.
What the Future Algorithm Will Look Like
Dr. Mayer presented a vision of how treatment sequencing may evolve:
- Endocrine therapy + CDK4/6 inhibitor remains the standard.
- ctDNA-triggered introduction of camizestrant + CDK4/6 inhibition may emerge as a new paradigm.
- SERD-based combinations (imlunestrant + abemaciclib; giredestrant + everolimus) and PI3K-pathway–directed strategies.
- PROTAC degraders and next-generation ER antagonists.
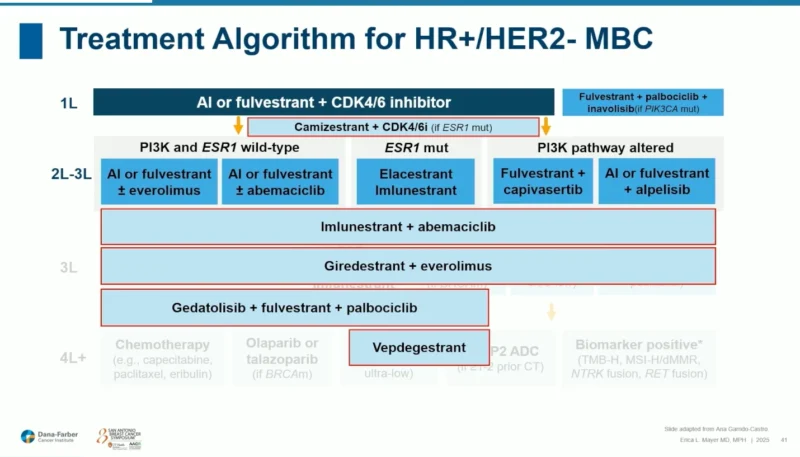
The landscape is becoming richer—but also more complex—requiring baseline and serial genomic profiling to guide choices.
For more information click here.


